You are here: Home > Section on Cellular Signaling
Signaling and Secretion in Neuroendocrine Cells

- Stanko S. Stojilkovic, PhD, Head, Section on Cellular Signaling
- Claudio E. Coddou Alvarez, PhD, Visiting Fellow
- Marek Kucka, PhD, Visiting Fellow
- Shuo Li, PhD, Visiting Fellow
- Melanija Tomic, PhD, Staff Scientist
- Zonghe Yan, MD, PhD, Research Fellow
Using multidisciplinary and collaborative approaches, the Section investigates signaling pathways at the cellular and molecular levels, determines the manner in which hormones and neurotransmitters utilize calcium as an intracellular messenger, and characterizes channels involved in electrical activity and calcium signaling in hypothalamic, pituitary, and other cell types. The Section has investigated cellular signaling cascades and secretion in neuroendocrine cells, with special emphasis on the interactions between plasma membrane electrical events and receptor-controlled pathways. Currently, we are investigating how the structural features of pituitary channels relate to the channels' functions and how plasma membrane receptors and the intracellular signaling milieu affect channel activity. For this purpose, we characterize native and recombinant channels and receptors that were cloned from the pituitary gland. We also analyze the relevance of these channels and pathways to calcium-dependent cellular processes.
Electrophysiological properties of pituitary and pineal cells
The membrane potential of isolated pituitary cells in vitro is not stable but oscillates from resting potentials of −65 to −50 mV, reflecting the balance between the activity of depolarizing and hyperpolarizing channels. It has been proposed that sodium conductance plays a role in the control of resting membrane potential, but the channel has not been identified. Several investigations have also suggested that the sodium-conducting channels are responsible for the cAMP-dependent facilitation of electrical activity and calcium influx. In collaboration with Constantine Stratakis' group, we studied dependence of spontaneous and cAMP–facilitated electrical activity of pituitary cells on a bath sodium. Because there is no common Gs–coupled receptor among these cells, we used forskolin, an activator of adenylyl cyclase. We used single-cell recordings to measure electrical activity, membrane currents, and intracellular calcium; we measured cyclic-nucleotide intracellular content and release in mixed pituitary cell populations. To distinguish a direct effect of cAMP on HCN channels from its indirect effect through a cAMP–dependent kinase (PKA), we used two inhibitors of HCN channels, ZD7288 and Cs, and two inhibitors of PKA, H89 and RP-cAMPs, on forskolin-stimulated electrical activity and calcium signaling. To directly investigate the role of PKA in the regulation of these channels, we also used two mouse models with altered PKA signaling: Prkar1a+/− and Prkar1a+/−Prkaca+/−.
Our results indicate that forskolin dose-dependently increases cAMP production and facilitates calcium influx in about 30% of rat and mouse pituitary cells at forskolin's maximal concentration. The stimulatory effect of forskolin on calcium influx was lost in cells in which PKA was inhibited and in cells that were haploinsufficient for the main PKA regulatory subunit but was preserved in cells that were also haploinsufficient for the main PKA catalytic subunit. Spontaneous and forskolin-stimulated calcium influx was present in cells with inhibited voltage-gated sodium and hyperpolarization-activated cation channels but was absent in cells bathed in medium in which sodium was replaced with organic cations. Consistent with the role of sodium-conducting nonselective cation channels in PKA–stimulated calcium influx, cAMP induced a slowly developing current with a reversal potential of about 0 mV. Two TRP (transient receptor potential) channel blockers, SKF96365 and 2-APB, as well as flufenamic acid, an inhibitor of nonselective cation channels, also inhibited spontaneous and forskolin-stimulated electrical activity and calcium influx. Quantitative RT–PCR analysis in rat pituitary cells indicated the following levels of expression of mRNA transcripts: TRPC1 >> TRPC6 > TRPC4 > TRPC5 > TRPC3. The experiments suggest that, in pituitary cells, constitutively active cation channels are stimulated further by PKA and contribute to calcium signaling indirectly by controlling the pacemaking depolarization in a sodium-dependent manner and directly by conducting calcium (1).
In our collaborative work with David Klein's group, we used perforated patch-clamp recording to study the control of membrane potential (Vm) and spontaneous electrical activity in the rat pinealocyte by norepinephrine. Norepinephrine did not alter spiking frequency. However, it was found to act through alpha1-adrenoreceptors in a concentration-dependent manner (0.1–10 μM) to produce a biphasic change in Vm. The initial response was a hyperpolarization (∼13 mV from a resting potential of −46 mV) due to a transient (∼5 sec) outward potassium current (∼50 pA). This current appears to be triggered by calcium released from intracellular stores, given that we also observed it in cells bathed in calcium-deficient medium. In addition, pharmacological studies indicate that this current was dependent on phospholipase C (PLC) activation and was in part mediated by bicuculline- and apamin-sensitive, calcium-controlled potassium channels. The initial transient hyperpolarization was followed by a sustained depolarization (∼4 mV) due to an inward current (∼10 pA), a response that was dependent on PLC–dependent activation of sodium/calcium influx but did not involve nifedipine-sensitive voltage-gated calcium channels. Together, these results indicate for the first time that activation of alpha1 adrenoreceptors initiates a PLC-dependent biphasic change in pinealocyte Vm characterized by an initial transient hyperpolarization mediated by a mixture of calcium-activated potassium channels followed by a sustained depolarization, which is mediated by a calcium-conducting nonselective cation channel. These observations indicate that both continuous elevation of intracellular calcium and sustained depolarization at approximately −40 mV are associated with, and are likely to be required for, activation of the pinealocyte (see Zemkova et al., Endocrinology 2011;152: 3842).
Gating properties and function of pituitary purinergic receptor channels
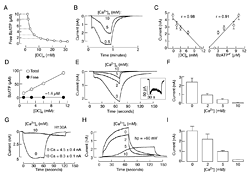
Click image to enlarge.
Figure 1. Dependence on extracellular calcium concentration of BzATP–induced P2X7R current
(A) Calculated free BzATP concentration in medium containing 100 µM BzATP and variable concentrations of calcium in the presence of 1 mM magnesium. Horizontal dotted lines indicate solutions used for activation of P2X7R shown in B and C. (B) Typical patterns of P2X7R current in response to application of 100 µM BzATP in medium containing 0.5, 5, and 10 mM calcium. (C) Correlation between divalent cation concentrations ([DC]e) and peak amplitude of current responses (left), and between free BzATP concentration and peak amplitude of current response (right). r, Correlation coefficient. (D-F) Variable current responses in cells stimulated with media containing identical free BzATP but variable calcium concentrations. (D) Comparable free BzATP concentrations achieved with variable total BzATP, 1 mM magnesium, and variable calcium concentrations. (E) A decrease in the amplitude of currents in response to media containing identical free BzATP concentrations (1.6 µM) but increasing calcium concentrations. Inset shows the current response to medium containing 10 mM calcium on an enlarged scale. (F) Mean ± SEM values of peak current amplitude measured 40 s after application of BzATP. (G) Effects of bath calcium on the peak amplitude of current in cells expressing the H130A-P2X7R mutant; representative traces (left) and mean ± SEM values from four experiments (right). (H and I) Concentration-dependent effects of bath calcium on the activation time and peak amplitude of current; representative traces (H) and mean ± SEM values from four experiments (I).
Purinergic P2X receptors (P2XRs) are ATP–gated cation channels. Seven mammalian receptor subunits, denoted P2X1 through P2X7, and several spliced forms of these subunits have been identified. Each subunit has only two transmembrane domains, the N- and C-termini facing the cytoplasm, and a large extracellular loop. The subunits assemble together as homo- or heterotrimers to make functional receptors. P2XRs likely have three intersubunit orthosteric binding sites located in the ectodomain, and their full occupancy appears to be required for conformational changes in the transmembrane domain channel gate, leading to facilitation of cation influx through the channel pore. All P2XRs are permeable to sodium, potassium, and calcium and some are also permeable to chloride. Positive and negative allosteric modulators of P2XRs interact with binding sites that are topologically distinct from the orthosteric sites recognized by the endogenous receptor agonist ATP, causing conformational changes that profoundly influence the gating of P2XRs (2).
The P2X7R is an unusual member of this family of channels. Structurally, the P2X7 subunit is distinguished from other subunits by its long intracellular C-terminal tail containing multiple protein and lipid interaction motifs and a cysteine-rich 18–amino acid segment and by its inability to make stable heteromeric complexes. It also appears that this channel exhibits various permeability states, which further complicates understanding of coupling of conformational changes in the orthosteric binding domains with the corresponding changes at the transmembrane channel gate. At least two conflicting hypotheses have been postulated to reconcile these findings. (i) The pore-dilation hypothesis suggests that there is a progressive dilation of the cation-conducting pore. (ii) The two-pore hypothesis implies activation of an endogenous P2X7R pore permeable to inorganic cations, accompanied by sustained activation of a distinct channel called pannexin, which is permeable to larger organic cations and fluorescent dyes (2).
Recently, we addressed both hypotheses. Our results indicate that naive receptors activated and deactivated monophasically at low and biphasically at higher agonist concentrations. Both phases of response were abolished by application of Az10606120, a P2X7R–specific antagonist. The slow secondary growth of current in the biphasic response coincided with pore dilation. Repetitive stimulation with the same agonist concentration caused sensitization of receptors, which manifested as a progressive increase in the current amplitude, accompanied by a slower deactivation rate. Once a steady level of the secondary current was reached, responses at high agonist concentrations were no longer biphasic but monophasic. Sensitization of receptors was independent of sodium and calcium influx, and about 30-minute washout was needed to reestablish the initial gating properties. T15E– and T15K–P2X7 mutants showed increased sensitivity to agonists, responded with monophasic currents at all agonist concentrations, activated immediately with dilated pores, and deactivated slowly. The complex pattern of gating exhibited by wild-type channels can be accounted for by a Markov state model, developed by our collaborators Anmar Khadra and Arthur Sherman, that includes negative cooperativity of agonist binding to unsensitized receptors caused by the occupancy of one or two binding sites, opening of the channel pore to a low conductance state when two sites are bound, and sensitization with pore dilation to a high conductance state when three sites are occupied (3).
We also combined biophysical and mathematical approaches to clarify the role of calcium in P2X7R gating. In naive receptors, bath calcium affected the activation permeability dynamics indirectly, by decreasing the potency of orthosteric agonists in a concentration-dependent manner and independently of the concentrations of the free acid form of agonists and status of pannexin1 channels (Figure 1). Bath calcium also facilitated the rates of receptor deactivation in a concentration-dependent manner, but did not affect a progressive delay in receptor deactivation caused by repetitive agonist application. The effects of calcium on the kinetics of receptor deactivation were rapid and reversible. A438079, a potent orthosteric competitive antagonist, protected the rebinding effect of BzATP on the kinetics of current decay during the washout period, but, in the presence of A438079, calcium also raised the rate of receptor deactivation. The corresponding kinetic model indicated that the decrease in binding affinity leads to a decrease in current amplitudes and facilitation of receptor deactivation, both in a manner dependent on extracellular calcium concentration, expressed as a Hill function. The results indicate that, at physiological concentrations, calcium acts as a negative allosteric modulator of P2X7R by lowering the affinity of receptors for orthosteric ligand agonists but not antagonists and not by affecting the permeability dynamics directly or indirectly, through pannexin-1 channels. We expect these results to generalize to other P2XRs (4).
Finally, in collaboration with Anne-Marie Heegaard, we analyzed the role of these channels in bone cancer pain. We demonstrated that P2X7R–knockout mice were susceptible to bone cancer pain and moreover had an earlier onset of pain-related behaviors than cancer-bearing, wild-type mice. Furthermore, acute treatment with the selective P2X7 receptor antagonist A-438079 failed to alleviate pain-related behaviors in models of bone cancer pain with and without astrocyte activation, suggesting that astrocytic P2X7R plays a negligible role in bone cancer pain. The results support the hypothesis that bone cancer pain is a separate pain state from those of neuropathic and inflammatory pain (Hansen et al., Pain 2011;152:1766).
Expression and roles of pannexins in ATP release in the pituitary gland
The physiological sources of the extracellular nucleotides required for activation of purinergic receptors in pituitary cells remain largely uncharacterized. It has been suggested that two members of the gap junction superfamily of proteins, connexins and pannexins, account for non-vesicular ATP release in other cell types. These proteins show identical membrane topology: four transmembrane domains connected by two extracellular loops and one intracellular loop with both N- and C-termini in the cytosol. The structure is essential for the formation of hexameric pore complexes termed hemichannels, which are large, non-selective ion channels expressed in the plasma membrane before their assembly into gap junctions. Pannexins are a three-member family of channels, termed Pannexins 1, 2, and 3. Unlike connexins, homomeric pannexin 1 hexamers do not form gap junctions when expressed in mammalian cells and, instead, operate as plasma membrane channels. They are activated, in a receptor-dependent manner, by mechanical stress and membrane depolarization. Pannexins are permeable to ions, small molecules, and metabolites up to 1 kDa, including NAD, cyclic nucleotides, and InsP3.
Recently, we showed that the rat pituitary gland expresses mRNA and protein transcripts of pannexins 1 and 2 but not of pannexin 3. Pannexin 1 is more abundantly expressed in the anterior lobe, whereas pannexin 2 is more abundantly expressed in the intermediate and posterior pituitary. We identified pannexin 1 in corticotrophs and a fraction of somatotrophs, in S100–positive pituicytes of the posterior pituitary, in AtT20 (mouse pituitary adrenocorticotropin-secreting cells), and in rat immortalized pituitary cells secreting prolactin, while we detected pannexin 2 in the S100–positive folliculostellate cells of the anterior pituitary, melanotrophs of the intermediate lobe, and vasopressin-containing axons and nerve endings in the posterior lobe. Overexpression of pannexins 1 and 2 did not affected P2X7R gating but enhanced the release of ATP into the extracellular medium, which was blocked by the gap junction inhibitor carbenoxolone. Basal ATP release in AtT20 cells was also suppressed by down-regulating the expression of endogenous pannexin 1 but not pannexin 2 with their short interfering RNAs. These results indicate that pannexins may provide a pathway for delivery of ATP, which is a native agonist for numerous P2X cationic channels and G protein–coupled P2Y receptors endogenously expressed in the pituitary gland (5).
Publications
- Tomic M, Kucka M, Kretschmannova K. Li S, Nesterova M, Stratakis CA, Stojilkovic SS. Role of nonselective cation channels in spontaneous and protein kinase A-stimulatred calcium signaling in pituitary cells. AM J Physiol Endocrinol Metab 2011;301:E370-E379.
- Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev 2011;63:641-683.
- Yan Z, Kadra A, Li S, Tomic M, Sherman A, Stojilkovic SS. Experimental characterization and mathematical modeling of P2X7 receptor channel gating. J Neurosci 2010;30:14213-14224.
- Yan Z, Khadra A, Sherman A, Stojilkovic SS. Calcium-dependent block of P2X7 receptor channel function is allosteric. J Gen Physiol 2011;138:437-452.
- Li S, Bjelobaba I, Yan Z, Kucka M, Tomic M, Stojilkovic SS. Expression and roles of pannexins in ATP release in the pituitary gland. Endocrinology 2011;152:2342-2352.
Collaborators
- Ivana Bjelobaba, PhD, University of Belgrade, Serbia
- Anne-Marie Heegaard, MD, PhD, The Danish University of Pharmaceutical Sciences, Copenhagen, Denmark
- J. Pablo Huidobro-Toro, PhD, Catholic University, Santiago, Chile
- Anmar Khadra, PhD, Laboratory of Biological Modeling, NIDDK, NIH
- David C. Klein, PhD, Program in Developmental Endocrinology and Genetics, NICHD, Bethesda, MD
- Arthur Sherman, PhD, Laboratory of Biological Modeling, NIDDK, NIH
- Constantine Stratakis, MD, D(med)Sci, Program in Developmental Endocrinology and Genetics, NICHD, Bethesda, MD
- Hana Zemkova, PhD, Institute of Physiology of the Academy of Science of the Czech Republic, Prague, Czech Republic
Contact
For more information, email stankos@helix.nih.gov or visit neuroscience.nih.gov/Faculty/Profile/stanko-stojilkovic.aspx.

