You are here: Home > Section on Membrane Biology
Membrane Rearrangements in Myoblast Fusion and in Cell Entry by Cationic Peptides

- Leonid V. Chernomordik, PhD, Head, Section on Membrane Biology
- Eugenia Leikina, DVM, Senior Research Assistant
- Kamram Melikov, PhD, Staff Scientist
- Elena Zaitseva, PhD, Research Fellow
- Samristha Sanyal, PhD, Visiting Fellow
- Santosh K. Verma, PhD, Visiting Fellow
- Ann Hara, BS, Postbaccalaureate Fellow
Disparate membrane remodeling reactions are tightly controlled by protein machinery but are also dependent on the lipid composition of the membranes. Whereas each kind of protein has its own personality, membrane lipid bilayers have rather general properties manifested by their resistance to disruption and bending. Our long-term goal is to understand how proteins remodel membrane lipid bilayers in important cell biology processes. We expect that the analysis of the molecular mechanisms of important and diverse membrane rearrangements will clarify the generality of emerging mechanistic insights. Better understanding of fusion mechanisms will bring about new ways of controlling them and lead to new strategies for quelling diseases involving cell invasion by enveloped viruses, intracellular trafficking, and intercellular fusion. Our general strategy is to combine in-depth analysis of the best characterized fusion reactions with comparative analysis of diverse, less explored fusion reactions, which can reveal new kinds of fusion proteins and mechanisms. In recent studies, we focused on the cell-to-cell fusion stage during development and regeneration of skeletal muscle and on lipid rearrangements underlying cell entry by cell-penetrating cationic peptides.
Extracellular annexins and dynamin are important for sequential steps in myoblast fusion.
Of three major families of biological membrane fusion processes—the fusion stage of virus entry, intracellular traffic, and cell-to-cell fusion—the latter remains the least explored. We still do not know the proteins that fuse cells in normal physiological processes (for instance, during fertilization) and in different pathological conditions (including carcinogenesis). In our recent studies, we focused on fusion between mammalian cells. Skeletal muscles are the most important example of cell fusion, as they constitute over 30 percent of human body weight. The importance of fusion in muscle development was suggested as early as 1839 by Theodor Schwann, who noted that “every primitive muscle bundle is a secondary cell formed by fusion of primary nuclei containing round cells.” While this hypothesis has been confirmed in many subsequent studies, the protein machinery that mediates this important cell-to-cell fusion process remains to be identified. A key challenge in studying the fusion stage of multinucleated syncytium formation is to isolate the fusion event per se from processes that prepare cells for fusion and to distinguish proteins that are required for fusion from those required for pre-fusion stages. For instance, fusion of myoblasts is preceded by differentiation of myoblast precursor cells, acquisition of fusion competence, and recognition and adhesion between myoblasts. Changes in the activities of proteins involved in any of these stages may result in fusion defects.
Click image to enlarge.
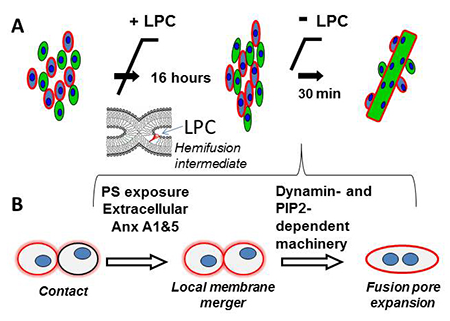
Figure 1. Schematic diagram that illustrates the experimental approach developed in (1) to uncouple fusion stage from pre-fusion processes (A) and the proposed pathway of the fusion stage of myotube formation (B)
(A) Myoblast fusion is blocked by LPC, a lipid that inhibits an early fusion intermediate hemifusion (= a local merger of contacting leaflets of two membrane bilayers). We accumulate ready-to-fuse-cells for 16 hours and then wash out LPC to release the block. (B) Cartoon that summarizes our findings on the fusion stage of myotube formation. We found that a local membrane merger at the onset of fusion involves extracellular Anxs A1 and A5 associated with the PS-exposing surface of fusion-committed myoblasts. Subsequent fusion stages (transition from a local membrane merger to complete unification of the volumes of fusing cells) are controlled by DNM– and PIP2–dependent intracellular protein machinery.
In our recent study (1), we analyzed in vitro myotube formation by C2C12 and primary mouse myoblasts. We used treatment with lysophosphatidylcholine (LPC) to uncouple the cell-to-cell fusion stage from the earlier stages of myogenesis that prepare the cells for fusion. As we had discovered earlier, LPC reversibly blocks the merger of the contacting leaflets of the fusing membranes at the onset of diverse membrane fusion processes, so that an LPC block allowed us to accumulate ready-to-fuse cells and to observe a relatively synchronized fusion upon LPC removal (Figure 1). Using this approach, we effectively separated the fusion stage from the upstream processes of myogenesis and thus concentrated within 30 minutes the fusion events that would normally develop within a 16-hour time span. To distinguish conditions that affect different stages of myoblast fusion, we measured both lipid mixing (observed already at early fusion stages) and syncytium formation (detected only in completed fusion events).
Earlier studies established the dependence of myotube formation on extracellular Ca2+ and on a transient phosphatidylserine (PS) exposure in the outer leaflet of the plasma membrane of fusion-committed myoblasts at cell–cell contact sites. These reports motivated us to explore whether synchronized myoblast fusion involves annexins (Anxs). Anxs are a large family of structurally related proteins whose common property is calcium-dependent binding to anionic phospholipids such as PS. While the exact biological functions of various Anxs remain to be clarified, proteins of this family have been implicated in many intracellular and extracellular processes, including exocytosis, plasma membrane repair, blood coagulation, apoptosis, adhesion, and inflammation. Intriguingly, Anxs A1 and A5 are transiently upregulated at the time of myotube formation in vitro and in vivo, and it has been reported that Anx A1 is involved in myogenic differentiation and myotube formation. We found that early stages of the synchronized fusion detected as lipid mixing between the cells do indeed involve Anxs A1 and A5. Antibodies to Anxs A1 and A5, and also peptides derived from the N-terminal domain of these Anxs, inhibit synchronized myoblast fusion and myotube formation. Myotube formation is also inhibited by siRNA suppression of Anxs A1 and A5 expression. Similarly, primary myoblasts isolated from either Anx A1–mutant or Anx 5–mutant mice are deficient for myotube formation in vitro. Reducing both Anx A1 and A5 together inhibits myoblast fusion more effectively than lowering the expression of either one of these Anxs alone. Fusion inhibition accomplished by lowering the concentration of one of these Anxs can be rescued by application of a recombinant version of either Anx A1 or Anx A5, suggesting that the functions of the two Anxs overlap and that the Anxs can substitute for each other in knockout mice lacking either Anx A1 or A5. The specific mechanisms by which extracellular Anxs and/or their protein partners form membrane connections between fusion-committed myoblasts remain to be clarified.
Early fusion stages (hemifusion and even opening of a fusion pore) are less energy-demanding than the subsequent expansion of nascent membrane connections within the cell contact region, and thus cell fusion does not always proceed to completion (i.e., syncytium formation). Mechanisms that drive the expansion of a fusion pore or pores to a micrometer-sized lumen, which allows complete coalescence of cytoplasms, are yet to be established for any cell fusion process. These late fusion stages can proceed either by vesiculation of the contact zone or by lateral displacement of the membrane material to the periphery of the fusion site. Both vesiculation and growth of the fusion pore within the tight contact zone accumulate the elastic energy of membrane bending. Intriguingly, we found that the transition from nascent membrane connections to multinucleated myotubes in the synchronized myoblast fusion involves membrane-bending dynamin (DNM) and is inhibited by lowering of the membrane concentration of accessible phosphatidylinositol 4,5-bisphosphate (PIP2), an important regulator of the membrane-bending proteins. Interestingly, some mutations in DNM and myotubularin, a protein involved in turnover of phosphoinositides, cause centronuclear myopathies characterized by an abnormal localization of cell nuclei; such mutations are possibly related to a delay in muscle fiber maturation.
To summarize, we dissected myoblast fusion into two distinct stages with an early stage of membrane merger involving extracellular Anx A1 and A5 and subsequent syncytium formation dependent on DNM activity and PIP2 content. Uncoupling fusion from preceding stages of myogenesis will help in: the analysis of the interplay between protein machines that initiate and complete cell unification; identification of additional protein players controlling different fusion stages; and, eventually, developing new ways of accelerating muscle regeneration after injuries and of treating degenerative muscle disorders.
Cationic cell-penetrating peptide nanoarginine does not induce conductive pores.
Macromolecular drugs such as polynucleotides, their mimics (peptide nucleic acids and phosphorodiamidate morpholino oligomers), and full-length proteins have great potential in the treatment of various diseases. Rapid development of such drugs is greatly facilitated by progress in biotechnology and by improved understanding of the molecular mechanisms of pathogenesis. However, delivery of macromolecular drugs to their intracellular targets remains challenging, because several permeability barriers have to be overcome, including a cell membrane that is generally impermeable to most macromolecules. Cationic cell-penetrating peptides (CPPs) are promising delivery vehicles for a wide range of potential macromolecular drugs and have been used successfully by many researchers to deliver various macromolecular cargo molecules both in vitro and in vivo.
Click image to enlarge.
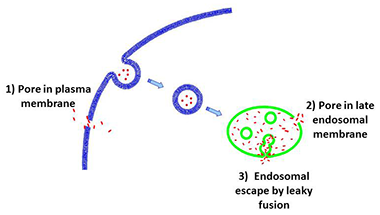
Figure 2. Suggested pathways of the cell entry by CPPs
CPP (red points) can enter the cells by forming pores in plasma membrane (route 1). Alternatively, CPP can be endocytosed and then escape from endosomes by forming pores in the endosomal membrane (route 2). In a third route, the peptides escape from endosomes by leaky fusion of internal vesicles in late endosomes. Our findings (3) argue against routes 1 and 2 but for the route 3.
While delivery vehicles such as oligoarginines are among the most extensively studied and practically used, the mechanisms by which these very polar molecules cross biological membranes remain poorly understood. It is possible that, similar to amphipatic antimicrobial peptides, CPPs can induce pores in plasma membrane (Figure 2, route 1) or, following endosomal uptake, in endosomal membrane (Figure 2, route 2). Indeed, several studies based on molecular simulations have substantiated the hypothesis that arginine-rich CPPs transfer directly across the plasma membrane by inducing ion-conducting pores. On the other hand, we and others have demonstrated that at physiological temperature CPPs are endocytosed before delivery into the cell cytosol and nucleus and thus have to escape from endosomes. Thus the question arises as to whether interaction of CPPs with membranes can lead to pore formation. To address this, we (in collaboration with the Sergey Bezrukov's lab) used highly sensitive electrical measurements on planar lipid bilayers that would detect a single pore with a 0.3-nm radius and a 1-ms lifetime (3). We observed neither CPP–induced single-channel activity nor any increase in background membrane conductivity for planar lipid bilayers formed from either uncharged lipids (in analogy to the outer leaflet of the plasma membrane) or negatively charged lipids (in analogy to late endosomal membranes). We found that neither the presence of CPP in solution nor its sorption to the membrane surface constitutes a sufficient condition for ion-conductive structure formation in neutral, negatively charged, positively charged, or asymmetrical membranes. Our new results along with the previously published data indicate that different cationic CPPs can induce formation of ion-conducting structures in membranes, but that such activity requires both the presence of anionic lipids in the membrane and CPP–dependent fusion of opposing membranes. We propose that, in a cellular context, CPP traffics to multivesicular late endosomes at physiological temperature. In the lumen of multivesicular late endosomes, CPPs bind to intraluminal vesicles enriched in negatively charged lipids. CPP–mediated leaky fusion between these vesicles allows peptide to enter the lumen of intraluminal vesicles (Figure 2, route 3). Finally, back-fusion of intraluminal vesicles with limiting membrane of late endosome releases CPP and associated cargo into the cytosol.
Additional Funding
- NIH's Intramural AIDS Targeted Antiviral Program (IATAP) 2013-2014
Publications
- Leikina E, Melikov K, Sanyal S, Verma SK, Eun B, Gebert C, Pfeifer K, Lizunov VA, Kozlov MM, Chernomordik LV. Extracellular annexins and dynamin are important for sequential steps in myoblast fusion. J Cell Biol 2013;200:109-123.
- Costin JM, Zaitseva E, Kahle KM, Nicholson CO, Rowe DK, Graham AS, Bazzone LE, Hogancamp G, Sierra MF, Fong RH, Yang S-T, Lin L, Robinson JE, Doranz BJ, Chernomordik LV, Michael SF, Schieffelin JS, Isern S. Mechanistic study of broadly neutralizing human monoclonal antibodies against dengue virus that target the fusion loop. J Virol 2013;87:52-66.
- Gurnev PA, Yang ST, Melikov KC, Chernomordik LV, Bezrukov SM. Cationic cell-penetrating peptide binds to planar lipid bilayers containing negatively charged lipids but does not induce conductive pores. Biophys J 2013;104:1933-1939.
Collaborators
- Sharon Isern, PhD, Florida Gulf Coast University, Fort Myers, FL
- Michael M. Kozlov, PhD, DHabil, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- Karl Pfeifer, PhD, Program in Genomics of Differentiation, NICHD, Bethesda, MD
- Félix Rey, PhD, Institut Pasteur, Paris, France
Contact
For more information, email chernoml@mail.nih.gov.

