You are here: Home > Section on Intercellular Interactions
Transmission and Pathogenesis of HIV-1 and its Copathogens in Human Tissues

- Leonid Margolis, PhD, Head, Section on Intercellular Interactions
- Jean-Charles Grivel, PhD, Staff Scientist
- Christophe Vanpouille, PhD, Staff Scientist
- Anush Arakelyan, PhD, Visiting Fellow
- Sonia Zicary, PhD, Visiting Fellow
- Andrea Introini, MS, PhD Student
- Wendy Fitzgerald, BS, Technician
Our general goal is to understand the mechanisms of pathogenesis and sexual transmission of human pathogens, including the human immunodeficiency virus (HIV). To achieve this, a comprehensive understanding of the mechanisms of the early events of HIV infection of human tissues is required, in particular of the lower female genital tract, where the critical events of this process occurs.
During the last year, we focused on three aims, to: (i) investigate the role of seminal cytokines in HIV-1 infected men in HIV-1 sexual transmission; (ii) develop tissue models in which to study these processes and to use these models to test new multi-targeted drugs to contain HIV-I infection and transmission; and (iii) design modern technologies to study antigenic composition of individual HIV virions.
During the past year, our research provided new insights into HIV-1 transmission and pathogenesis, leading to new concepts in anti-HIV-1 strategies. In particular, we found that one of the major seminal cytokines in HIV-1–infected men facilitates virus transmission to a cervico-vaginal ex vivo tissue system. The system reflects many important aspects of the in vivo situation and is now further benchmarked by our studies on the dependence of HIV transmission to the menstrual phase.
We use the system of cervico-vaginal tissue ex vivo in continuation of our translational research and to test the new dual-targeted anti-HIV/anti-herpesvirus (HHV) anti-herpetic drugs.
Understanding the basic mechanisms of infection by HIV and other pathogens requires the development of new experimental and diagnostic technologies. We report on one of them, which is based on nanotechnology. Specifically, we developed a new technology to characterize the antigenic composition of individual HIV virions. The new technology may reveal which of the HIV virions are the most transmittable/pathogenic so that the new anti-HIV strategies could specifically target those virions.
Seminal cytokines in male-to-female HIV-1 transmission
The majority of HIV-1 infections in women occur through vaginal intercourse, during which virus-containing semen is deposited on the cervico-vaginal mucosa. Semen is more than a mere carrier of HIV-1, since it contains many biological factors, in particular cytokines, that may affect HIV-1 transmission. The cytokine interleukin 7 (IL-7) belongs to a family of proteins that regulate the immune response. IL-7 is present in normal semen and is found at especially high levels in the semen of men with HIV. We investigated the potential role of IL-7 in HIV-1 vaginal transmission in an ex vivo system of human cervico-vaginal tissue. To achieve this, we developed a culture system of cervico-vaginal explants and used it to simulate male-to-female transmission of HIV. In particular, we deposited HIV-1 on cervico-vaginal tissue in combination with IL-7 at concentrations comparable with those measured in semen of HIV-1–infected individuals. We found that IL-7 significantly enhanced virus replication in ex vivo infected cervico-vaginal tissue. Similarly, we observed an enhancement of HIV-1 replication in lymphoid tissue explants. IL-7 significantly enhanced replication of the R5 variant HIV-1BaL in cervico-vaginal explants from all the donors tested in this study, including one tissue sample in which there was no replication in the untreated tissue.
Similarly, we observed an enhancement of HIV-1 replication in lymphoid tissue infected with both R5 and X4 HIV-1 variants, given that, unlike cervico-vaginal tissue ex vivo, lymphoid tissue supports replication of HIV-1 of both phenotypes. The enhancement became detectable on day six post infection and appeared to be independent of the absolute level of viral replication, as it was also observed in tissues infected with a viral inoculum diluted 100 fold. The enhancement of HIV-1 replication by IL-7 was not restricted to laboratory strains of this virus but was also observed for primary isolates. Moreover, we observed the enhancement for HIV-1 isolates of all three tested clades. Among these isolates were mono CCR5-tropic and dual CXCR4/CCR5-tropic HIV-1 variants. Viruses of the former tropism are thought to be preferentially transmitted and to dominate early stages of infection, while the latter are characteristic of later stages of HIV-1 disease. Thus, IL-7 appears to be an enhancer of replication of different HIV-1 variants replicating at different levels in different human tissues. Analysis of T cells isolated from infected tissues showed that IL-7 reduced CD4+ T cell depletion, thus preventing apoptosis, as shown by the lower number of cells expressing the apoptotic marker APO2.7 and rise in the expression of the anti-apoptotic protein B-cell lymphoma (Bcl)-2. Also, IL-7 raised the fraction of cycling CD4+ T cells, as evidenced by staining for the nuclear factor Ki-67. High levels of seminal IL-7 in vivo may be relevant to the survival of the founder pool of HIV-1–infected cells in the cervico-vaginal mucosa at the initial stage of infection, promoting local expansion and dissemination of HIV infection.
The finding raises the possibility that IL-7, alone or in combination with other molecules, can foster male-to-female transmission of HIV. Moreover, the level of IL-7 in semen may determine how infectious a particular HIV-positive male is for a female sexual partner. Also, it is possible to use IL-7 as a new target for preventive anti-HIV strategy.
Benchmarking of the system of cervico-vaginal tissue ex vivo and testing new antivirals
As stated above, most newly HIV type-1 (HIV-1)–infected women acquire infection through vaginal intercourse in which semen-associated virus is deposited in the mucosa of the lower female genital tract. In order to reach its primary cell targets in the female lower genital tract, the virus must circumvent mucosal barriers. The mucosal barrier components as well as the state of activation of HIV-1 target cells are strongly influenced by the menstrual cycle, which is under the control of sexual hormones. In order to study this influence and to further “benchmark” it, we used the system of cervico-vaginal tissue explants, which retain the in vivo cyto-architecture and some tissue functions.
Cervical tissue explants (CTE) from 22 HIV-1 seronegative women were exposed to R5 HIV-1 ex vivo. Eight CTEs were productively infected in terms of HIV-1 p24Gag release in culture supernatants, whereas 14 were not. Nonetheless, both accumulation of HIV-1gag DNA and of p24Gag+ CD4+ T cells and macrophages occurred in both productive and, at lower levels, in nonproductive CTEs. Nonproductive CTEs differed from productive CTEs for higher secretion of C-C motif chemokine ligand 3 (CCL3) and CCL5. A post-hoc analysis revealed that all productive CTEs were established from women in the secretory phase of their menstrual cycle, whereas nonproductive CTEs were derived from women either in their secretory (28%) or proliferative (36%) menstrual cycle phases or with an atrophic endometrium (36%). Thus, our results demonstrate an association between the capacity of the cervical tissue to support productive HIV-1 infection, at least ex vivo, and the menstrual cycle phase of the donor, in particular with the progesterone-dependent secretory phase. Our study also emphasizes that the system of cervico-vaginal tissue ex vivo faithfully reflects epidemiologic observation on the dependency of HIV transmission on the menstrual cycle and thus represents an appropriate experimental system in which to study the mechanisms underlying the different susceptibility to HIV-1 infection in different stages of the menstrual cycle as well as other aspects of HIV infection.
In particular, the system is now used as a platform to study new antivirals. We continued to use it to develop dual-targeted antivirals against HIV and herpesviruses (HHV). Indeed, HIV infection is often accompanied by infection with other pathogens, in particular herpes simplex virus type 2 (HSV-2). The resulting coinfection is involved in a vicious circle of mutual facilitations. Therefore, an important task is to develop a compound that is highly potent against both viruses to suppress their transmission and replication. We developed such a compound, an acyclic nucleoside phosphonate designated PMEO-DAPym. We compared its properties with those of the structurally related and clinically used acyclic nucleoside phosphonates (ANPs) tenofovir and adefovir. We demonstrated the potent anti-HIV and anti-HSV activity of this drug. PMEO-DAPym markedly inhibits both HIV-1 reverse transcriptase (RT) and HSV DNA polymerase. We did not limit testing of this compound to cervico-vaginal tissue ex vivo, but also confirmed its anti HIV/HHV activity in several other experimental systems.
In conclusion, we defined a distinct new subclass of acyclic nucleoside phosphonates, structurally and functionally distinct from the earlier developed and widely used tenofovir and adefovir, that provides significant advantage over the commonly used drugs.
Flow virometry
Click image to enlarge.
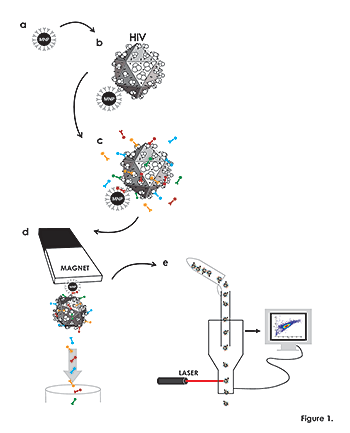
Figure 1. Flow virometry procedure
(a) Magnetic nanoparticles (MNPs) coupled to a virion-specific antibody against gp120 (“capture antibody”), (grey). (b) MNPs coupled to capture antibody are incubated with viruses (schematically presented as icosahedrons), which become immobilized on MNPs. (c) MNP–immobilized viruses are visualized with human anti-gp120 antibody (blue) recognizing an epitope different from the one recognized by the capture antibody. MNPs are visualized with a fluorescent antibody or its Fab (red) against the Fc portion of the capture antibody, and viral antigens of interest are visualized with fluorescently labeled monoclonal antibodies (green and yellow). (d) Virus–MNP complexes with bound antibodies are separated from unbound antibodies on magnetic columns. (e) The MNP–immobilized virions stained with fluorescence-labeled antibodies eluted from the magnetic columns are analyzed with the flow cytometer set up to trigger on MNP fluorescence rather than on light scatter.
About 50 years ago, flow cytometry revolutionized immunology in particular and medicine in general, and, by making possible the distinction of individual cells by their antigenic spectra, gained unprecedented new insights into mechanisms of immunity, including responses to infection by viruses. Unlike lymphocytes, viruses themselves are still characterized predominantly in bulk, in spite of the strong indications that viral particles are as individualized as lymphocytes. Because of its high mutation rate, HIV-1 is one of the most diverse human viruses.
We developed “flow virometry”, a new technology that, by using magnetic nanopatrticles, allows antigen detection on individual virions. The technology consists of binding magnetic nanoparticles to virions, staining virions with monoclonal antibodies, separating the formed complexes on magnetic columns, and characterizing the virions with flow cytometers. Using this technology, we studied the distribution, among HIV-1 virions, of two antigens (HLA-DR and LFA-1) that HIV-1 acquires from infected cells. Unlike techniques reported earlier, flow virometry allows one not only to demonstrate the presence of these antigens on viruses but, importantly, to analyze the distribution of antigens among individual viral particles.
We evaluated the distribution of HLA-DR and LFA-1 on two HIV-1 variants replicated in two different cell preparations. In the antigenic makeup of virions from a single preparation, our results revealed high antigenic heterogeneity, which cannot be detected with bulk analysis of viruses, the only method currently available. Moreover, in two preparations of the same HIV-1, but produced by different cells, the distribution of antigens among virions was different. In contrast, HIV-1 of two different HIV-1 genotypes replicating in the same cells became somewhat antigenically similar. The new nanotechnology is not restricted to the analysis of HIV but can be applied to the analysis of the individual antigenic makeup of any virus. The new method is a technical advance that may give new insights into basic mechanisms of viral infection and may lead to the development of new more precisely targeted antivirals.
Additional Funding
- Intramural-to-Russia (I-to-R) Program
Publications
- Lisco A, Munawwar A, Introini A, Vanpouille C, Saba E, Feng X, Grivel J, Singh S, Margolis LB. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis 2012;205:97-105.
- Lisco A, Introini A, Munawwar A, Vanpouile C, Grivel J-C, Blank P, Singh S, Margolis LB. HIV-1 imposes rigidity on blood and semen cytokine network. Am J Reprod Immunol 2012;68:515-521.
- Introini A, Vanpouille C, Lisco A, Grivel J-C, Margolis L. Interleukin-7 facilitates HIV-1 transmission to cervico-vaginal tissue ex vivo. PLoS Pathogens 2013;9:1-10.
- Saba E, Origoni M, Taccagni G, Ferrari, D, Doglioni C, Nava A, Lisco A, Grivel J-C, Margolis L, Poli G. Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol 2013;Epub ahead of print.
- Arakelyan A, Fitzgerald W, Margolis L, Grivel J-C. Flow virometry: a nanoparticle based technology for analysis of individual viral particles. J Clin Invest 2013;123:3716-3727.
Collaborators
- Jan Balzarini, PhD, Rega Institute, Katholieke Universiteit Leuven, Leuven, Belgium
- Sarman Singh, MD, All India Institute of Medical Sciences, New Delhi, India
Contact
For more information, email margolis@helix.nih.gov.

