You are here: Home > Unit on Cellular Communication
Mechanisms of Cellular Communication During Development

- Mihaela Serpe, PhD, Head, Unit on Cellular Communication
- Mikolaj Sulkowski, PhD, Postdoctoral Fellow
- Tae Hee Han, PhD, Visiting Fellow
- Cathy Ramos, PhD, Visiting Fellow
- Qi Wang, PhD, Visiting Fellow
- Grace Kim, BS, Postbaccalaureate Fellow
- Peter Nguyen, Biological Laboratory Technician
Synapse development is initiated by genetic programs but is coordinated by intercellular communication between the pre- and postsynaptic compartments and by neuronal activity itself. Understanding this coordination is the central objective of our research. Synaptogenesis requires three key processes: (i) trafficking the components to the proper site; (ii) organizing those components to build synaptic structures; and (iii) maturation and homeostasis of the synapse to optimize its activity. Our long-term goal is to dissect the central role of cellular signaling in these processes and the molecular mechanisms by which it does so, using the Drosophila neuromuscuar junction (NMJ) as a model system for glutamatergic synapse development and, as our entry point, the ionotropic glutamate receptor (iGluR) auxiliary protein Neto.
The Drosophila NMJ is an ideal system in which to study mechanisms of synapse assembly and homeostasis. The fact that individual NMJs in flies can be reproducibly identified from animal to animal and are easily accessible for electrophysiological and optical analysis makes them uniquely suited for in vivo studies on synapse assembly, growth, and plasticity. In addition, the great richness of genetic manipulations that can be performed in Drosophila permits independent control of individual synaptic components in distinct cellular compartments. Lastly, the fly NMJ is a glutamatergic synapse similar in composition and physiology to mammalian central synapses. The Drosophila NMJ can thus be used to analyze and model defects in the structural and physiological plasticity of glutamatergic synapses, which are associated with a variety of human pathologies from learning and memory deficits to autism. The similarity in gross architecture, function, and molecular machinery supports the notion that studying the assembly and development of fly glutamatergic synapses will shed light on their vertebrate counterparts.
We recently discovered a novel protein, Neto (neuropillin and tolloid-like), that is essential for functional receptors. Our findings provide an entry point to understand the molecular mechanisms of synapse assembly and development.
Drosophila NMJ, a well established model to study synapse development
L-glutamate is the primary neurotransmitter at excitatory synapses in the vertebrate CNS and at arthropod NMJs. However, the molecular mechanisms that trigger the recruitment of glutamate receptors and promote their stabilization at postsynaptic densities (PSDs) remain poorly understood.
In flies as in vertebrates, before a muscle is innervated, low levels of postsynaptic neurotransmitter receptors are present diffusely in the muscle membrane. Once the motor-neuron growth cone arrives at its target muscle, the postsynaptic receptors begin to concentrate at the synaptic cleft. The mechanisms by which neuronal arrival regulates clustering of postsynaptic nicotinic acetylcholine receptors at the vertebrate NMJ are relatively well understood. Clustering and stabilization of glutamate receptors at postsynaptic locations remain the focus of intense research. Live studies at the Drosophila NMJ demonstrated that glutamate receptor clusters are immobilized at PSDs in large, stable aggregates that grow up to a clearly defined maximum size. The NMJ grows by adding new synaptic contacts and by enlarging the existing synapses to maximum size. During development, several trans-synaptic signals coordinate the growth of synaptic structures. At the Drosophila NMJ, the signals include an anterograde Wnt signaling pathway and a retrograde BMP signaling pathway. Furthermore, a retrograde signal ensures constant excitability/synapse homeostasis by increasing the presynaptic release of neurotransmitter-filled vesicles in response to diminished postsynaptic sensitivity. Although the nature of this retrograde signal remains to be determined, the homeostatic response can only be triggered when the BMP signaling pathway components are in place.
We focus on several key questions in neurobiology: (i) how iGluRs traffic to the synapse; (ii) how they form stable clusters at the mature PSDs; (iii) the nature of the neuronal cue that triggers iGluRs clustering at the onset of synaptogenesis; (iv) how cellular communication balances synapse activity with synapse growth and maturation.
Drosophila Neto, an essential auxiliary subunit required for functional receptors
Drosophila iGluRs are heterotetrameric complexes composed of three shared subunits, GluRIIC, GluRIID and GluRIIE, and either GluRIIA (type-A receptors) or GluRIIB (type-B). The shared subunits are essential for viability—without any of them the animals are completely paralyzed and die as late embryos. The receptor subunits are also dependent on each other for synaptic recruitment. We recently discovered Neto as an essential auxiliary protein absolutely required for clustering and stabilization of iGluRs at the Drosophila NMJ. Neto belongs to a family of highly conserved proteins sharing an ancestral role in formation and modulation of glutamatergic synapses. Live-imaging studies show that Neto clusters at nascent NMJs at the time when iGluRs begin to accumulate and cluster. neto–null mutants are completely paralyzed and die as embryos, similar to animals lacking iGluRs. Consistent with this observation, we found that, in neto–null embryos, the iGluR subunits do not cluster at synaptic locations and remain scattered as small aggregates, away from the neuronal arbor. Both the lethality and iGluR clustering defects of neto–null mutants can be rescued by restoring Neto activity postsynaptically in the muscle. Importantly, Neto does not cluster at synaptic locations in the absence of iGluRs. Our studies indicate that Neto forms a complex with the iGluRs on the muscle membrane and that the Neto/iGluRs complexes traffic together to synaptic sites, where they form clusters. By controlling the clustering and trafficking of functional iGluR complexes, Neto directly controls synapse assembly, organization and maintenance of PSDs, and synapse functionality.
The Neto proteins are multidomain, transmembrane proteins with two extracellular CUB (for complement C1r/C1s, UEGF, BMP-1) domains followed by an LDLa (low-density lipoprotein receptor domain class A) motif. CUB domains are BMP–binding, protein-interaction domains that could promote aggregation via self-association and/or extracellular interactions. CUB–containing proteins have been implicated in formation of multi-molecular complexes, including acetylcholine receptor aggregates in C. elegans.
Mapping functional domains of Neto
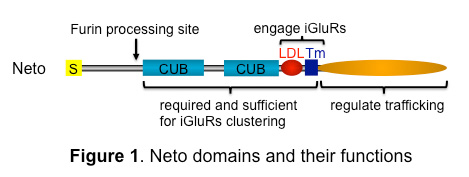
Figure 1. Neto domains and their functions
Neto proteins have highly conserved extracellular domains, including two CUB domains and an LDLa motif, a single transmembrane pass, and variably intracellular parts. In insects, Neto has an additional prodomain followed by a Furin processing site.
To characterize the functional domains of Neto, we generated truncated Neto variants and tested their cellular distribution and ability to rescue Neto function during development. We found that the extracellular part of Neto is required for apical targeting as well as for clustering at the NMJ (Figure 1). The intracellular domains of Neto could be completely removed without preventing targeting and clustering at the NMJ. However, a point mutation in the cytoplasmic domain traps Neto at extra-junctional locations, suggesting a regulatory role for the domain in Neto trafficking. We also determined that Neto binds to iGluRs via its LDLa motif and transmembrane domain (Tm). Our studies indicate (a) that the extracellular and Tm part of Neto are both required and sufficient to (i) interact with iGluRs, (ii) target Neto to postsynaptic sites, and (iii) stabilize iGluRs/Neto clusters at the PSDs; and (b) that the intracellular domains regulate the synaptic targeting of Neto/iGluR complexes.
Neto does not appear to influence the iGluRs surface delivery. To test whether the extrajunctional iGluRs are on the surface of the postsynaptic muscle, we developed antibodies against the GluRIIC extracellular domain and stained fillets of the third instar in the absence of detergents. The GluRIIC signals were present on the surface of the muscle irrespective of Neto levels, suggesting that Neto does not influence the surface localization of GluRIIC.
Neto prodomain removal enables iGluR stabilization and postsynaptic differentiation.
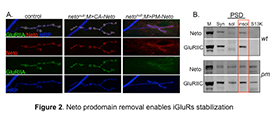
Figure 2. Neto prodomain removal enables iGluRs stabilization.
A. Confocal images of NMJ4 boutons in larvae of indicated genotypes labeled for Neto (red), GluRIIA (green), and HRP, which marks the neuron surface (blue). Muscle expression of CA-Neto rescues the NMJ defects of neto–null mutants. In contrast, PM-Neto–rescued NMJs have aberrant synapses with impaired iGluRs distribution.
B. Distribution of Neto and GluRIIC was analyzed in synaptosomal (syn) and PSDs fractions from the body wall muscles of control and PM-Neto–rescued larvae. Neto and GluRIIC were restricted to the insoluble (insol) PSD fraction in control, but partitioned to both soluble (sol) and insoluble fractions in the muscle of PM-Neto–rescued animals.
Analysis of the Neto prodomain reveals a requirement for Neto in iGluR stabilization and initiation of postsynaptic differentiation. Neto activities are restricted by an inhibitory prodomain, which must be removed by Furin-mediated proteolysis. When the prodomain cleavage is blocked (such as in the processing mutant PM-Neto), Neto is properly targeted to the muscle membrane and engages the iGluR complexes in vivo, as found in co-immunoprecipitation analysis. However, the PM-Neto–rescued neto–null animals die mostly during larval stages and have severely impaired NMJs and reduced iGluR synaptic clusters (Figure 2). In contrast, a constitutively active Neto (CA-Neto) efficiently rescues the viability and iGluR–clustering defects of neto–null mutants.
Stable synaptic receptors are thought to be part of large aggregates organized by presynaptically secreted components and stabilized by postsynaptic scaffolds. To test whether Neto modulates iGluR incorporation into synaptic aggregates, we isolated and analyzed synaptosome-enriched fractions from third instar larval body wall muscles. Both Neto and the iGluRs are found mostly in the insoluble, PSD–enriched fraction in controls. But in fractions isolated from PM-Neto–rescued animals, PM-Neto predominantly partitions to the more soluble fractions. The distribution of GluRIIC closely follows the Neto patterns, indicating that the removal of Neto prodomain is required for the stabilization of iGluRs at PSDs.
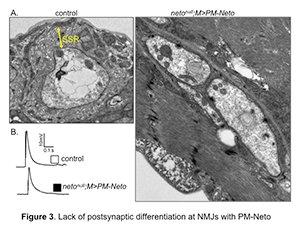
Intriguingly, the instability of PM-Neto/iGluR complexes does not impair their synaptic activity; these channels are functional and have relatively normal evoked potentials owing to a robust presynaptic compensatory response. However, NMJs containing these receptor complexes lack any distinguishable postsynaptic structures, as if their postsynaptic differentiation was never initiated (Figure 3). Thus, synapse activity alone does not trigger postsynaptic differentiation; instead, Neto-dependent stabilization of iGluRs at synaptic locations initiates an active process of recruitment of PSD components and assembly of postsynaptic structures. Although the molecular nature of the stabilization mechanism(s) remains to be determined, our results make it clear that the process requires the removal of the Neto prodomain, presumably to unmask its protein-interaction domains.
Figure 3. Lack of postsynaptic differentiation at NMJs with PM-Neto
A. Electron micrographs of synaptic boutons from control and PM-Neto–rescued third instar larvae. Postsynaptic structures, such as sub synaptic reticulum (SSR), are densely packed around control boutons, but are lacking at PM-Neto–rescued boutons.
B. Representative traces of evoked (excitatory junction potential–EJP) neurotransmitter release recorded from muscle 6 of indicated genotypes. The EJPs measured at PM-Neto NMJs are largely normal, indicating that iGluRs stabilization is not required for neurotransmission.
Synaptic pMad as a molecular sensor of synapse activity
At the Drosophila NMJ, retrograde BMP signaling is critical for synapse growth and homeostasis. Retrograde BMP signaling is carried by Glass bottom boat (Gbb), a BMP–type ligand secreted by the muscle, which binds to heteromeric (type I and type II) receptor complexes on the motor neuron surface. Binding of Gbb induces accumulation of the pathway effector, the phosphorylated Smad (pMad), in motor neuron nuclei and at synaptic locations. Nuclear pMad, in conjunction with other transcription factors, modulates expression of BMP target genes and promotes synaptic growth. In the absence of BMP signaling, the NMJs remain small and have reduced excitability. The biological relevance of synaptic pMad remained a mystery for over a decade.
We found that pMad signals are selectively lost at Neto–deprived NMJ synapses. In contrast, nuclear pMad persists in motor neuron nuclei, and expression of BMP target genes is unaffected, indicating a specific impairment in pMad production/maintenance at synaptic terminals. Given that BMP signaling is generally short lived and Mad does not appear to traffic together with the BMP/BMP receptor complexes to the neuron cell body, synaptic pMad likely reflects accumulation of active BMP signaling complexes at synaptic terminals. Furthermore, synaptic pMad mirrors the synaptic accumulation of postsynaptic Neto/iGluRs complexes. In fact, pMad exclusively follows the postsynaptic type-A receptor subtypes and tracks their activity (the quantal size) but not the net levels of postsynaptic type-A receptors. Therefore, synaptic pMad could serve as an exquisite monitor for synapse activity.
In recent work, we found that the local BMP complexes are different from the transcriptionally active BMP complexes, which are retrogradely transported and utilized in the neuron soma. Also, different, genetically distinguishable mechanisms modulate these canonical and non-canonical BMP pathways. Some of the pathway components are shared, thus limiting, suggesting a coordinated role for BMPs in monitoring postsynaptic activity and transcriptional control of target genes in the presynaptic compartment.
Publications
- Sulkowski M, Kim YJ, Serpe M. Postsynaptic glutamate receptors regulate local BMP signaling at the Drosophila neuromuscular junction. Development 2014;436-447.
- Wharton K, Serpe M. Fine-tuned shuttles for bone morphogenetic proteins. Curr Opin Genet Dev 2013;23:374-384.
- Kim YJ, Serpe M. Making a synapse: a complex matter. Fly (Austin) 2013;7:146-152.
Collaborators
- Seth S. Blair, PhD, University of Wisconsin, Madison, WI
- Chi-Hon Lee, MD, PhD, Program in Cellular Regulation and Metabolism, NICHD, Bethesda, MD
- Mark Mayer, PhD, Program in Developmental Neuroscience, NICHD, Bethesda, MD
- Bing Zhang, PhD, University of Oklahoma, Norman, OK
Contact
For more information, email serpemih@mail.nih.gov or visit ucc.nichd.nih.gov.