You are here: Home > Section on Developmental Genetics
Heritable Neurodegenerative, Inflammatory, and Autoimmune Disorders

- Anil Mukherjee, MD, PhD, Head, Section on Developmental Genetics
- Goutam Chandra, PhD, Visiting Fellow
- Sondra W. Levin, MD, Adjunct Scientist
- Matthew Moralle, BS, Postbaccalaureate Fellow
- Shiyong Peng, PhD, Visiting Fellow
- Arjun Saha, PhD, Visiting Fellow
- Chinmoy Sarkar, PhD, Visiting Fellow
- Zhongjian (Gary) Zhang, MD, PhD, Staff Scientist
The Section on Developmental Genetics conducts both laboratory and clinical investigations to understand the molecular mechanisms of pathogenesis of genetic disorders of neurodegeneration, inflammation, and autoimmunity. These studies are conducted in order to develop novel therapeutic strategies for diseases for which currently there is no effective treatment. Our current focus is on the regulation and physiological functions of two genes: (1) palmitoyl-protein thioesterase-1 (Ppt1), mutations of which cause infantile neuronal ceroid lipofuscinosis (INCL), commonly known as infantile Batten disease, a devastating neurodegenerative storage disorder of childhood; and (2) that encoding uteroglobin (UG), also known as Clara cell 10 kDa protein (CC10), which manifests potent anti-inflammatory, immunomodulatory, and anti-chemotactic properties. We have generated UG-knockout (UG-KO) mice, which develop IgA-nephropathy, pulmonary inflammation, and susceptibility to tumorigenesis and metastasis. Our investigations into both genes have led to ongoing clinical trials. In ongoing investigations with UG, we discovered that this protein manifests properties that suppresses lung metastasis of certain cancer cells.
Using PPT1-knockout (PPT1-KO) mice, which recapitulate virtually all clinical and pathological features of INCL, we discovered that deficiency in this enzyme leads to endoplasmic reticulum and oxidative stresses, which at least in part mediate neuronal apoptosis, a suggested cause of neurodegeneration in INCL. We also delineated the mechanism by which PPT1-deficiency disrupts the recycling of the synaptic vesicle (SV) proteins, essential for the regeneration of fresh SVs to replenish the SV-pool size at the nerve terminals required for maintaining uninterrupted neurotransmission. These and other related discoveries provide insight into the complex mechanism of neurodegeneration in INCL and identify several potential therapeutic targets. Our ongoing laboratory and clinical investigations continue to break new ground in the hope of finding new treatments for the above mentioned diseases.
ER and oxidative stresses are common mediators of apoptosis in lysosomal storage disorders and are alleviated by chemical chaperones
More than 40 lysosomal storage disorders (LSD) cumulatively affect 1 in 5,000 live births. Neurodegeneration is a prominent feature of the majority of these disorders. As a group, neuronal ceroid lipofuscinoses (NCLs) represent one of the most common (1 in 12,500 births) neurodegenerative LSDs. The infantile NCL, or INCL, is the most devastating neurodegenerative LSD. We previously reported that ER and oxidative stresses, at least in part, cause neuronal death by apoptosis in both INCL and in PPT1-KO mice that mimic INCL. Over the past year, we unexpectedly found that ER and oxidative stresses are not unique manifestations of INCL but are common to both neurodegenerative and non-neurodegenerative LSDs. Moreover, cells from several LSDs studied in our laboratory show extraordinary sensitivity to brefeldin A–induced apoptosis, suggesting pre-existing ER stress conditions. Further, we found that chemical disruption of lysosomal homeostasis in normal cells causes ER stress, suggesting a cross-talk between lysosomes and the ER. Most importantly, we found that chemical chaperones, which alleviate ER and oxidative stresses, are also cytoprotective in all forms of LSDs studied. We propose that ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative LSDs, and that alleviation of these stress conditions by chemical/pharmacological chaperones may have beneficial effects.
Neuroinflammation caused by cytosolic PLA2 activation contributes to neuropathology in INCL
In the majority of neurodegenerative storage disorders, neuronal death in the brain is followed by infiltration of phagocytic cells (e.g., activated microglia, astroglia, and macrophages) for the efficient removal of dead cells. However, it is increasingly evident that the phagocytes may also cause death of adjoining viable neurons, thus contributing to rapid progression of neurodegeneration. PPT1 catalyzes the cleavage of thioester linkages in S-acylated (palmitoylated) proteins while a deficiency in the enzyme leads to abnormal accumulation of thioesterified polypeptides (ceroid) in lysosomes, thereby causing INCL pathogenesis. As noted, PPT1-KO mice mimic the clinical and pathological features of human INCL, including rapid neuronal death by apoptosis and phagocyte infiltration. We previously reported that, in PPT1-KO mice, neurons undergo ER stress, activating the unfolded protein response, which mediates caspase-12 activation and apoptosis. However, the molecular mechanism(s) by which the phagocytic cells are recruited in the PPT1-KO mouse brain remains poorly understood. We found that increased production of lysophosphatidylcholine (LPC), catalyzed by the activation of cytosolic phospholipase A2 (cPLA2) in the PPT1-KO mouse brain, is a “lipid signal” for phagocyte recruitment. We also report that an age-dependent increase in LPC levels in the PPT1-KO mouse brain positively correlates with elevated expression of the genes characteristically associated with phagocytes. We propose that increased cPLA2-catalyzed LPC production in the brain is at least one of the mechanisms that mediates phagocyte infiltration contributing to INCL neuropathology.
A bench-to-bedside clinical trial: effects of a combination of Cystagon™ and Mucomyst® on INCL patients
INCL is caused by inactivating mutations in the lysosomal Ppt1 gene. Given that PPT1 catalyzes the cleavage of thioester linkages in palmitoylated (S-acylated) proteins (see above), its deficiency impairs hydrolysis of these lipid-modified proteins by lysosomal proteases. Consequently, accumulation of these lipid-modified proteins (ceroid) in lysosomes leads to INCL pathogenesis. Because thioester linkages are susceptible to nucleophilic attack, drugs with this property may have therapeutic potential for INCL. We showed that two drugs, phosphocysteamine and N-acetylcysteine, cleave thioester linkages in [14C]palmitoyl-CoA, a model substrate of PPT1, and in lymphoblasts and fibroblasts from INCL patients. These drugs also facilitated the depletion of lysosomal ceroids and inhibited apoptosis. These results prompted us to initiate a bench-to-bedside clinical protocol to determine whether a combination of Cystagon™ (cysteamine bitartrate) and Mucomyst® (N-acetylcysteine) is beneficial for patients with INCL. Our protocol was approved by the NICHD-IRB for the treatment of 20 INCL patients who carry the most lethal mutations in the Ppt1 gene. So far, we have treated 10 INCL patients; this study is currently ongoing and will continue until the approved number of patients are treated. The protocol currently admits INCL patients who are 6 months to 3 years of age. Patients may be referred to Dr. Mukherjee at mukherja@exchange.nih.gov.
Children with infantile Batten disease (INCL) have an increased risk of hypothermia and bradycardia during anesthesia.
Even though rare (1 in >100,000 births), infantile Batten disease, also know as INCL, is the most lethal among all the NCL subtypes. INCL is caused by mutations in the gene CLN1, which encodes palmitoyl-protein thioesterase-1 (PPT-1). During a bench-to-bedside clinical trial we observed that some children undergoing general anesthesia for head MRI to evaluate neurodegeneration, developed hypothermia and bradicardia. To investigate their incidence during general anesthesia in patients with INCL, we conducted a case-control study. Eight children with INCL (mean age 25 months; [range, 10-32 months] at first anesthetic) and 25 controls (mean age 44 months [range, 18-92 months]) underwent 62 anesthesias for nonsurgical procedures. Patients with INCL had neurologic deficits including developmental delay, myoclonus, and visual impairment. They exhibited lower baseline temperature (36.4 ± 0.1 vs. 36.8 ± 0.1, INCL versus controls, P < 0.007), and during anesthesia, despite active warming techniques, and experienced significantly more hypothermia (18 vs. 0 episodes, P < 0.001) and sinus bradycardia (10 vs. 1, P < 0.001) than controls. We conclude that INCL patients are at significant risk for temperature decreases during anesthesia (P < 0.001), whereas age, sex, and duration of anesthesia were not (P = NS), suggesting a previously unknown INCL phenotype—impaired thermoregulation. We propose that during administration of anesthesia to these children, careful monitoring and routine use of warming interventions be used.
Subdural fluid collections in patients with INCL
Mutations in eight different genes cause neuronal ceroid lipofuscinoses (NCLs). As stated above, INCL is caused by mutations in Ppt1. Children afflicted with this disease are normal at birth, but by two years of age they undergo complete retinal degeneration, and by age four brain activities become undetectable. Currently, there is no treatment for INCL and it remains a uniformly fatal disease. During an ongoing bench-to-bedside clinical investigation, MRI examinations led to the incidental discovery of subdural fluid collections in four of nine patients with INCL. No particular event (such as trauma) or change in symptoms was linked to this finding, which was already in the chronic phase when discovered. Of the four patients, one was followed for 7 years, two for 4 years and a fourth patient was followed for 2.5 years. Over time, these collections remained stable or decreased in size. Recognition that subdural fluid collections are part of the INCL disease process may obviate the necessity of additional workup as well as therapeutic interventions in these chronically sick children.
Abnormal synaptic vesicle recycling contributes to neuropathology in INCL
The ER stress caused by PPT1 deficiency at least in part mediates neuronal apoptosis and contributes to neurodegeneration in INCL. Although INCL patients show signs of abnormal neurotransmission as manifested by myoclonus and seizures, the molecular mechanisms by which PPT1 deficiency causes this abnormality remain obscure. Neurotransmission relies on repeated cycles of exo- and endocytosis of synaptic vesicles (SVs) in which several palmitoylated proteins play critical roles. These proteins facilitate membrane fusion, which is required for neurotransmitter exocytosis, recycling of the fused SV membrane components, and regeneration of fresh vesicles. However, palmitoylated proteins require depalmitoylation for recycling. Using postmortem brain tissues from an INCL patient and tissue from PPT1-KO mice that mimic INCL, we found that PPT1 deficiency caused persistent membrane anchorage of the palmitoylated SV proteins, hindering the recycling of the vesicle components that normally fuse with the presynaptic plasma membrane during SV exocytosis. Thus, the regeneration of fresh SVs, essential for maintaining the SV pool size at the synapses, was impaired, leading to a progressive loss of readily releasable SVs and abnormal neurotransmission. This abnormality may contribute to INCL neuropathology.
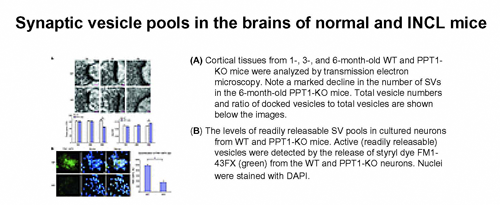
Figure 1. Progressive decline of the synaptic vesicle pool size in the mouse model of INCL
Lack of uteroglobin in mice promotes pulmonary metastasis of B16F10 melanoma cells
A link between inflammation and cancer metastasis has long been suspected but its molecular mechanism(s) remains largely unclear. The potent anti-inflammatory protein UG is constitutively expressed in the lungs of all mammals. UG-KO mice, which are susceptible to pulmonary inflammation, and B16F10 melanoma cells, which preferentially metastasize to the lungs, provide components of a model system to uncover how inflammation and metastasis are linked. We reported that the lungs of UG-KO mice express high levels of two chemokines, S100A8and S100A9, while B16F10 cells express elevated levels of RAGE (receptor for advanced glycation end products), which also serves as the receptor for these chemokines. RAGE on B16F10 cells, which interacts with S100A8/S100A9 present in UG-KO mouse lungs, may create a pro-inflammatory microenvironment that promotes metastasis. We demonstrated that S100A8/S100A9 are potent chemoattractants for B16F10 cells in vitro and that pre-treatment of these cells with a blocking antibody to RAGE suppresses their chemotactic migration. Moreover, we found a concentration gradient of S100A8/S100A9, from the tail vein (low) to the lungs (high) of UG-KO mice, which most likely provides a roadmap for B16F10 cells to migrate to this organ. Most notably, treatment of B16F10 cells with recombinant S100A8 or S100A9 stimulates the expression of matrix metalloproteinases (MMPs) and furin, a pro-protein convertase that activates MMPs, which are known to confer the invasiveness essential for cancer metastasis. Our results define a mechanism by which lack of an anti-inflammatory protein promotes metastasis of cancer cells to the lungs and identify RAGE as a potential drug-target.
Publications
- Kim SJ, Zhang Z, Sarkar C, Tsai PC, Lee YC, Dye L, Mukherjee AB. Palmitoyl protein thioesterase-1 deficiency impairs synaptic vesicle recycling at nerve terminals, contributing to neuropathology in humans and mice. J Clin Invest 2008 118:3075-3086.
- Levin SW, Baker E, Gropman A, Quezado ZMN, Miao N, Zhang Z, Jollands A, Di Capua M, Mukherjee AB. Patients with infantile neuronal ceroid lipofuscinosis are susceptible to developing subdural fluid collections. Arch Neurol 2009 [in press].
- Miao N, Levin SW, Baker EH, Caruso RC, Zhang Z, Gropman A, Koziol D, Wesley R, Mukherjee AB, Quezado ZM. Children with infantile neuronal ceroid lipofuscinosis have an increased risk of hypothermia and bradycardia during anesthesia. Anesth Analg 2009 109:372-378.
- Wei H, Kim S-J, Zhang Z, Tsai PC, Wisniewski KE, Mukherjee AB. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum Mol Genet 2008 17:469-477.
- Zhang Z, Butler JD, Levin SW, Wisniswski K, Brooks SS, Mukherjee AB. A novel approach towards treatment of infantile neuronal ceroid lipofuscinosis, a hereditary progressive encephalopathy of childhood. Nat Med 2001 7:478-484.
Collaborators
- Eva Baker, MD, PhD, Clinical Center, NIH, Bethesda, MD
- Louis Dye, BS, Microscopy and Imaging Core Facility, NICHD, Bethesda, MD
- Andrea Gropman, MD, Georgetown University, Washington, DC
- Alan Koretsky, PhD, NMR Center, NINDS, Bethesda, MD
- Sondra W. Levin, MD, Walter Reed Army Medical Center, Washington, DC
- Ning Miao, MD, Clinical Center, NIH, Bethesda, MD
- Jeeva Munasinghe, PhD, NMR Center, NINDS, Bethesda, MD
- Zenaide Quezado, MD, Clinical Center, NIH, Bethesda, MD
- Yi-Jin Xiao, PhD, Cleveland Clinic, Cleveland, OH
- Yan Xu, PhD, Indiana University Medical School, Indianapolis, IN
Contact
For more information, email mukherja@exchange.nih.gov.



