You are here: Home > Unit on Growth and Obesity
Physiology, Psychology, and Genetics of Obesity

- Jack A. Yanovski, MD, PhD, Head, Unit on Growth and Obesity
- Diane C. Adler-Wailes, MS, Biologist
- Asem Ali MD, Clinical Fellow, Endocrine Training Program
- Sheila M. Brady, RN, FNP, Nurse Practitioner
- Melissa K. Crocker, MBA, MD, Clinical Fellow, Endocrine Training Program
- Shannon Fuhr, BA, Postbaccalaureate Fellow
- Joan C. Han, MD, Senior Clinical Endocrine Fellow
- Caroline A. Roza, BS, Postbaccalaureate Fellow
- David M. Savastano, PhD, Postdoctoral Fellow
- Lauren B. Shomaker, PhD, Special Volunteer
- Mahsa Sorouri, BS, Postbaccalaureate Fellow
- Babette Stern, BS, Postbaccalaureate Fellow
- Jaclyn Zocca, BA, Postbaccalaureate Fellow
The prevalence of overweight and obesity in children and adults has tripled during the past 30 years. The alarming rise in body weight has likely occurred because the current environment affords easy access to calorie-dense foods and requires less energy expenditure. However, the same environment leads to obesity only in those individuals whose body weight–regulatory systems are not able to control body adiposity with sufficient precision in our high-calorie/low-activity environment, thus suggesting that some subgroups in the United States have a uniquely high susceptibility to weight gain. Our goal is to elucidate the genetic underpinnings of the metabolic and behavioral endophenotypes that contribute to the development of obesity in children. Using our unique longitudinal cohort of children at risk for adult obesity, we examine genetic and phenotypic factors predictive of progression to adult obesity in children in the “pre-obese” state, allowing characterization of phenotypes unconfounded by the impact of obesity itself. We focus on genetic variants linked to obesity that impair gene function. We expect our approaches to improve our ability to predict which children are at greatest risk for obesity and its co-morbid conditions and to lead to more targeted, etiology-based prevention and treatment strategies for pediatric obesity.
Molecular studies of factors important for childhood body weight regulation
To identify gene variants impacting body composition, we have been examining polymorphisms in genes involved in the leptin signaling pathway. Genes include proopiomelanocortin (POMC), the melanocortin 3 receptor (MC3R), brain-derived neurotrophic factor (BDNF) FTO, and those encoding histaminergic receptors 1 and 3. We previously studied genes important for energy expenditure, such as those encoding the mitochondrial uncoupling proteins and genes potentially involved in cortisol metabolism that may impact intra-abdominal adipose tissue, such as 11-beta-hydroxysteroid dehydrogenase. We are currently studying a variant MC3R that is associated with adiposity in children and which appears to have functional significance for MC3R signal transduction. Children who were homozygous variant for both the polymorphisms Thr6Lys and Val81Ile had significantly greater BMI-SD score, fat mass, body circumference measurements, and higher plasma levels of insulin and leptin than chindren who did not bear the mutation or than heterozygous children. In vitro studies subsequently found that signal transduction and protein expression were significantly lower for the double mutant MC3R (Figure 1). In our ongoing studies, we are attempting to understand the mechanisms by which these sequence alterations may impact body weight. We recently found that children homozygous variant for both the polymorphisms Thr6Lys and Val81Ile showed no deficits in energy expenditure but demonstrated hyperphagia in laboratory meal studies—results that were specific to function-altering mutations and not associated with other common polymorphisms we identified in MC3R. Transgenic “knock-in” mice expressing the human wild-type and human double-mutant MC3R have been developed in collaboration with Heiner Westphal, and will be studied during the next two years.
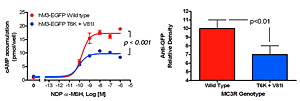
Click image to enlarge.
Figure 1.
Studies of a human MC3R variant containing two naturally occurring polymorphisms—a variant was associated with pediatric-onset obesity—found that the variant was partially inactive, with decreased signal transduction (left panel), likely due to reduced protein expression (right panel).
We have also recently investigated the BDNF-TrkB pathway as it relates to body mass in children. We measured serum BDNF in 328 children, age 3 to19, many of whom suffered from extreme obesity. BDNF was significantly lower in overweight children (mean ±SD, 39.8±24.8 vs. 47.0±25.4 ng/dL, p=0.03); in multiple regression analyses with log-BDNF as the dependent variable, BMI (p=0.03), BMI-Z (p=0.01), and body fat (p’s<0.02), were all negatively associated with BDNF. These data suggest that some obese individuals with low serum BDNF for age and platelet count may have mutations that alter BDNF function. In collaboration with Sadaf Farooqi and Stephen O’Rahilly, we found that individuals with heterozygous TrkB mutations do not have unusually high circulating BDNF, and we reported one child with hyperphagia and severe obesity associated with functional loss of expression from one copy of the BDNF gene. This child had a de novo chromosomal inversion, 46,XX,inv(11)(p13p15.3), a region encompassing the BDNF gene. The patient’s genomic DNA was heterozygous for a common coding polymorphism in BDNF but we found monoallelic expression in peripheral lymphocytes. Serum BDNF was markedly lower than in age- and BMI-matched control subjects. Functional haploinsufficiency for BDNF was thus associated with increased ad libitum food intake and severe early onset obesity. These findings provide evidence for the role of BDNF in human energy homeostasis.
A new initiative has assessed the role of BDNF haploinsufficiency as a cause of obesity in patients with syndromes that are due to deletions in the vicinity of 11p14.1, where the human BDNF gene is found. Using a comparative genomic hybridization approach that was verified with PCR-based assays, we are examining genotype-phenotype relationships in patients with 11p deletion syndromes such as the WAGR (Wilms Tumor, Aniridia, Genitourinary, and Renal abnormalities) syndrome. In 33 subjects with heterozygous 11p deletions ranging in size from 1.0-26.5 Mb, 19 had regions of deletion that involved the BDNF gene (BDNF+/−). Those with BDNF+/− had significantly greater body mass during childhood, starting at age 2y (Figure 3), than those with intact BDNF (BDNF+/+). 100% (95% CI: 76.8-100%) of BDNF+/− children were overweight by age 10 vs. only 20% (95%CI: 2.5-55.6%) of BDNF+/+ children (p<0.0001). Parent-completed hyperphagia questionnaires suggest significantly greater hyperphagic behavior, drive, and severity for BDNF+/− than for BDNF+/+ children. Mean serum BDNF was approximately 50% lower among BDNF+/− children (p=0.001). This result remained statistically significant after adjustment for sex, current age, BMI, and platelet count. We then examined the centromeric and telomeric boundaries of each individual’s deletion. There was no association between the extent of subjects’ centromeric deletion and childhood overweight. However, analysis of the telomeric deletion boundaries indicated the presence of a critical region for pediatric-onset overweight within 80 kb of the BDNF exon (Figure 3). One overweight subject had deletion of BDNF exons I through III but preservation of subsequent BDNF exons. Each subject who maintained normal weight during childhood had a deletion that did not involve BDNF. These findings suggest that BDNF haploinsufficiency in patients with WAGR syndrome leads to pediatric-onset obesity. A clinical protocol admitting patients with WAGR syndrome and other 11p deletion syndromes for a full characterization of their energy intake and expenditure is now ongoing. A second research direction in collaboration with Lino Tessarollo and Bai Liu involves characterizing the body composition and energy homeostasis of mice with selective replacement of several of the BDNF promoter regions that create unique BDNF splice variants with GFP. We hypothesize that mice lacking splice variants created by early promoter regions (I through III) will show obesity, while later promoters will be of less importance for hypothalamic BDNF function.

Click image to enlarge.
Figure 3.
Patients with WAGR Syndrome who have haploinsufficiency for brain-derived neurotrophic factor (BDNF) had higher BMI standard deviation score (BMI Z-Score) than children and adults with WAGR Syndrome who retained two copies of BDNF. Deletions that extended into exon 1 of BDNF were associated with 100% risk of childhood-onset obesity.
Physiology, metabolism, and psychology of childhood body weight regulation
Our studies are directed at understanding the physiological, psychological, and metabolic factors that place children at-risk for undue weight gain. As part of these studies, we have examined how best to measure eating-related psychopathology, insulin sensitivity, changes in body composition, energy intake, and energy expenditure in children. We have found that leptin is an important predictor of weight gain in children: in a longitudinal study, we found that those with high leptin experience accelerated weight gain. Recent investigations have also documented how hyperinsulinemia is related to energy intake. 95 non-diabetic, overweight (BMI≥95th percentile) children (age 10.3±1.4y) selected lunch from a 9,835kcal buffet (Figure 2) eaten ad libitum after an overnight fast. We determined the associations between energy intake and measures of insulin dynamics, in the post-absorptive state and during a 2h-hyperglycemic clamp. Energy intake was positively associated with the fasting homeostasis model assessment for insulin resistance index (HOMA-IR; beta = 0.24, p=0.042), fasting insulin/glucose ratio (beta = 0.24, p=0.044), firstt-phase insulin (beta = 0.23, p=0.032), and first-phase C-peptide (beta = 0.21, p=0.046); energy intake was negatively associated with clamp-derived insulin sensitivity (SIclamp; beta = 0.29, p=0.042). Each 10% decrease in SIclamp predicted 27 kcal greater energy intake. These associations suggest mechanisms whereby insulin resistance may contribute to excessive weight gain in children and have informed some of our treatment approaches to pediatric obesity (described below).
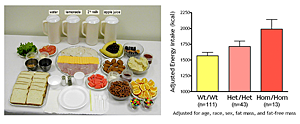
Click image to enlarge.
Figure 2.
Energy intake is studied using free access buffet meals of palatable foods. Children homozygous for two polymorphisms in the MC3R consumed more at the buffet than heterozygotes or those who had wild-type MC3R.
Our evaluations of binge eating behaviors in children suggest that such behaviors also are associated with adiposity in children. We have also found that binge eating and dieting behaviors may predict future weight gain in children at risk for overweight. Over an observation period of 4.2±1.8 years, children reporting binge-eating behaviors gained, on average, an additional 15% more fat mass than non-binge-eating children. Our recent data also suggest that children endorsing binge eating consume more energy during meals. Actual intake during buffet meals averaged 400 kcal more in children with binge eating, but despite their greater intake, such children reported shorter-lived satiety (194.2±84 vs. 262.1±89 min; p=0.03) than children without binge eating episodes. The ability to consume large quantities of palatable foods, especially when coupled with decreased subsequent satiety, may play a role in the greater weight gain found in binge eating children. These data also suggest that interventions targeting disordered eating behaviors may potentially be useful in preventing excessive fat gain in children prone to obesity.
In two ongoing protocols, we are studying normal-weight children and adolescents, children who are already obese, and the non-obese children of obese parents, in order to determine the factors that are most important for development of the complications of obesity in youth. We examine body composition, metabolic rate, insulin sensitivity, glucose disposal, energy intake at buffet meals, and genetic factors believed to regulate metabolic rate and body composition. Psychological and behavioral factors, such as propensity to engage in binge-eating behavior, are also studied. We are studying children longitudinally into adulthood. In two protocols, we are measuring actual food consumption of children during meals, to elucidate differences in the calorie and macronutrient content of meals and the circulating hormones related to hunger and satiety in those who either endorse binge eating behaviors or report no such behaviors. We hypothesize that differences in these factors predict the development of obesity in the populations studied and may be of great importance in developing rational approaches for the prevention and treatment of obesity in the diverse US population. A new clinical protocol based on a successful pilot study is examining the effects of a targeted interpersonal therapy intervention on body weight change in adolescents who endorse binge eating behaviors.
Treatment of obesity and the co-morbid conditions associated with obesity
Given the rapid increase in the prevalence of obesity, the development of treatments for obesity in children and adults is urgently needed. In four clinical protocols, we have examined approaches for the prevention and treatment of excessive body weight. We completed a pilot study demonstrating that severely overweight adolescents can lose weight when enrolled in a comprehensive weight management program that includes the gastrointestinal lipase inhibitor orlistat as an adjunct to a behavioral modification program. We recently completed a placebo-controlled randomized trial, studying whether the use of orlistat 120 mg TID improved the weight loss of African American and Caucasian children and adolescents who have obesity-related comorbidities. Subjects participated in a 12-week weight-reduction program. We compared body weight and body composition (by DXA and air displacement plethysmography), glucose homeostasis (by frequently sampled intravenous glucose tolerance test—FSIGT), fasting lipids, pulse, and blood pressure before and after treatment. We studied 200 adolescents, 65% female, 61% African American, mean age±SEM 14.6±0.10y, BMI 41.7±0.6 kg/m2 (range 27-87 kg/m2). At baseline, subjects randomized to orlistat (n=100) or placebo (n=100) did not differ significantly in age, sex, race, BMI, or fat mass, (all p>0.45). Of the subjects, 85.5% completed the trial. Adolescents treated with orlistat lost more weight (orlistat −2.9±0.7 vs. placebo −0.6±0.7 kg, p=0.011), BMI units (−1.72±0.24 vs. −0.70±0.24 kg/m2, p=0.002), and fat mass (−3.9±0.8 vs. −1.4±0.8 kg, p=0.029). Although pulse and blood pressure decreased during the trial (p<0.001), orlistat treatment did not significantly alter pulse or blood pressure (all p>0.25). Similarly, HOMA-IR, SI by FSIGT, Apo B, total and LDL-cholesterol, and triglycerides decreased in proportion to weight loss (p<0.001), but orlistat use was not associated with significant reductions in any of these obesity-related laboratory comorbidities (all p>0.2). Orlistat treatment did not significantly alter levels of the fat-soluble vitamins 25OH vitamin D, vitamin A, and vitamin E (all p>0.46). Both AST (+1.8±0.9 vs. −1.08±0.9, p=0.024) and ALT (+1.3±1.1 vs. −2.4±1.2, p=0.022) unexpectedly increased significantly with orlistat treatment. We concluded that, when added to a behavioral program, orlistat significantly improved weight loss over a 6-month interval but had little impact on obesity-related co-morbid conditions in overweight adolescents.
A second now-completed study examined the mechanism by which metformin may affect the body weight of younger children who have hyperinsulinemia and are therefore at risk for later development of type 2 diabetes. We conducted a single-center, 6-month, randomized, double-blind, placebo-controlled trial of the effects of metformin, 1000 mg BID, administered with meals, in severely overweight children (6-12y) who manifested hyperinsulinemia and insulin resistance. Subjects participated in a monthly dietitian-administered weight reduction program. Body mass index and body composition (by air displacement plethysmography), glucose homeostasis (by HOMA-IR), and lipids were measured before and after 6 months’ treatment. We enrolled 100 overweight children (60% female, 11% Hispanic, 3% Asian, 40% African American), mean age 10.2±1.5y, with mean BMI 34.6±6.6 kg/m2 (range 23-58 kg/m2) between October, 2000, and April, 2007. Eighty-five percent of subjects (84% given metformin and 86% given placebo) completed the trial. Children randomized to metformin experienced a significantly greater decreased in BMI (metformin −0.91±0.3 vs. placebo +0.23±0.3 kg/m2, p=0.006), BMI-Z score (−0.11±0.02 vs. −0.04±0.02, p=0.02) and body fat mass (−1.4±0.7 vs. +2.1±0.7 kg, p<0.001) than to placebo-treated children. Serum glucose (−2.4±0.9 vs. +1.6±1.2 mg/dL, p=0.018), HOMA-IR (−0.19±0.4 vs. +0.95±0.4, p=0.05), and total cholesterol (−9.9±2.7 vs. +1.1±4.8, p=0.04) also decreased more in metformin-treated than in placebo-treated children. We concluded that metformin, added to a monthly behavioral program, significantly improved weight loss and reduced insulin resistance and cholesterol over a 6-month interval in severely overweight, insulin-resistant children.
A third clinical trial in adults examined the role dietary calcium plays in body weight. We randomized 340 overweight (BMI 25 to <30 kg/m2) and obese (BMI ≥30 kg/m2) adults to take calcium carbonate (elemental calcium, 1500 mg/d, n = 170) or placebo, n = 170) with meals for two years. Seventy-five percent of participants completed the trial (78% received calcium; 73% received placebo). There were no statistically or clinically significant differences between the calcium and placebo groups in change in body weight (difference, 0.02 kg, 95% CI, −1.64 to 1.69 kg; p=0.98), BMI (difference, 0.32 kg/m2, CI, −0.41 to 1.02 kg/m2; p=0.39), or body fat mass (difference, 0.39 kg, CI, −1.04 to 1.92 kg; p=0.55). Parathyroid hormone concentrations decreased in the calcium group compared with the placebo group (difference, −0.71 pmol/L, CI, −1.28 to −0.13 pmol/L). We concluded that dietary supplementation with elemental calcium, 1500 mg/d, for two years had no statistically or clinically significant effects on weight in overweight and obese adults. Calcium supplementation is unlikely to have clinically significant efficacy as a weight-gain preventive measure in such patients.
A fourth ongoing study examines the role central nervous system histamine plays in controlling food intake at meals. Subjects are randomized to take placebo or one of several doses of betahistine, and we measure food intake at meals.
Additional Funding
- NIH Clinical Center “Bench to Bedside” Award: FTO and Eating in Absence of Hunger 2009-2011
- NIH Clinical Center “Bench to Bedside” Award: Histaminergic Pathways and Energy Intake in Obese Women 2008-2010
Publications
- Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, Adler-Wailes DC, Sanford EL, Lacbawan FL, Uhl GR, Rennert OM, Yanovski JA. Brain derived neurotrophic factor and obesity in WAGR syndrome. New Engl J Med 2008 359:918-927.
- Han JC, Rutledge MS, Kozlosky M, Salaita CG, Gustafson JK, Keil MF, Fleisch AF, Roberts MD, Ning C, Yanovski JA. Insulin resistance, hyperinsulinemia, and energy intake in overweight children. J Pediatr 2008 152:612-617.
- Yanovski JA, Parikh SJ, Yanoff LB, Denkinger BI, Calis KA, Reynolds JC, Sebring NG, McHugh T. Effects of calcium supplementation on body weight and adiposity in overweight and obese adults: a randomized trial. Ann Intern Med 2009 150:821-829.
- Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, Kozlosky MS, Schvey NA, Shomaker LB, Salaita C, Yanovski JA. Laboratory assessment of the food intake of children and adolescents with loss of control eating. Am J Clin Nutr 2009 89:738-745.
- Savastano DM, Tanofsky-Kraff M, Han JC, Ning C, Sorg RA, Roza CA, Wolkoff LE, Jefferson-George KS, Figueroa RE, Sanford EL, Brady S, Kozlosky MS, Schoeller DA, Yanovski JA. Energy intake and energy expenditure among children with polymorphisms of the melanocortin-3 receptor. Am J Clin Nutr 2009 90:912-920.
Collaborators
- Greti Aguilera, MD, Program in Developmental Endocrinology and Genetics, NICHD, Bethesda, MD
- Kong Chen, PhD, Clinical Endocrinology Branch, NIDDK, Bethesda, MD
- Christopher Cox, PhD, The Johns Hopkins University, Baltimore, MD
- Myles Faith, PhD, University of Pennsylvania School of Medicine, Philadelphia, PA
- I. Sadaf Farooqi, MD, Cambridge Institute for Medical Research, Cambridge, UK
- Oksana Gavrilova, PD, Mouse Metabolism Core Laboratory, NIDDK, Bethesda, MD
- Alexander Gorbach, PhD, Laboratory of Bioengineering and Physical Science, NIBIB, Bethesda, MD
- Van S. Hubbard, MD, PhD, Division of Nutritional Research Coordination, NIDDK, Bethesda, MD
- Joel E. Kleinman, MD, PhD, Clinical Brain Disorders Branch, NIMH, Bethesda, MD
- Rudolph L. Leibel, MD, Columbia University College of Physicians and Surgeons, New York, NY
- Bai Lu, PhD, Program in Developmental Neuroscience, NICHD, Bethesda, MD
- Stephen O’Rahilly, MD, Cambridge Institute for Medical Research, Cambridge, UK
- Dale A. Schoeller, PhD, University of Wisconsin, Madison, WI
- Marian Tanofsky-Kraff, PhD, USUHS Department of Psychology, Bethesda, MD
- Lino Tessarollo, PhD, NCI Frederick Mouse Cancer Genetics Program, Frederick, MD
- James Troendle, PhD, Division of Epidemiology, NICHD, Bethesda, MD
- George R. Uhl, MD, PhD, Molecular Neurobiology Branch, NIDA, Baltimore, MD
- B. Timothy Walsh, PhD, Columbia University College of Physicians and Surgeons, New York, NY
- Heiner Westphal, MD, Program in Genomics of Differentiation, NICHD, Bethesda, MD
- Denise E. Wilfley, PhD, Washington University School of Medicine, St. Louis, MO
- Alexander F. Wilson, PhD, Inherited Disease Research Branch, NHGRI, Baltimore, MD
- Susan Z. Yanovski, MD, Obesity and Eating Disorders Program, NIDDK, Bethesda, MD
Contact
For further information, contact yanovskj@mail.nih.gov or visit ugo.nichd.nih.gov.



