You are here: Home > Section on Molecular Endocrinology
Receptors and Actions of Peptide Hormones and Regulatory Proteins in Endocrine Mechanisms

- Maria L. Dufau, MD, PhD, Head, Section on Molecular Endocrinology
- Chon-Hwa Tsai-Morris, PhD, Staff Scientist
- Mingjuan Liao, PhD, Staff Fellow
- Junghoon Kang, PhD, Postdoctoral Fellow
- Raghuveer Kavarthapu, PhD, Postdoctoral Fellow
- Joaquin Villar, PhD, Postdoctoral Fellow
- Lisheng Dai, Predoctoral Fellow
We investigate the molecular basis of peptide hormone control of gonadal function, with particular emphasis on the structure and regulation of the luteinizing hormone(LHR) and prolactin (PRL) receptor (PRLR) genes. We also investigate the regulatory mechanism(s) involved in the progress of spermatogenesis and the control of Leydig cell function. Our studies focus on the regulation of human LHR transcription (nuclear orphan receptors, epigenetics, DNA methylation, and second messengers, repressors, corepressors, and coactivators), as well as on the multiple-promoter control of hPRLR gene transcription. We are elucidating the functions of two inhibitory short forms of the prolactin receptors and their impact on the long form of the receptor and their relevance to physiological regulation and breast cancer. We also investigate novel gonadotropin-regulated genes of relevance to the progression of testicular gametogenesis, Leydig cell function, and other endocrine processes. We concentrate our studies on the function and regulation of gonadotropin-regulated testicular RNA helicase (GRTH/DDX25), an essential post-transcriptional regulator of spermatogenesis that was discovered, cloned, and characterized in our laboratory. The various functions of GRTH/DDX25 provide a fertile ground for the development of a male contraceptive.
The luteinizing hormone receptor (LHR)
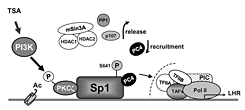
Click image to enlarge.
Figure 1. Model of derepression/activation of the LHR receptor gene transcription by histone deacetylase inhibitor TSA
Complex regulation that releases inhibitors factors and recruitment of positive PC4 cofactor cooperatively contributes to transcriptional activation of LHR transcription. Sp1 interacts directly or indirectly with repressor/corepressor factors including the HDAC1/2/mSin3A corepressor complex, the p107 repressor protein, and the serine/threonine phosphatases PP2A and PP1. Release of of such inhibitory complex/molecules is dependent on phospatase release induced by local chromatin changes and PI3K/PKCζ–mediated Sp1 phosphorylation at Ser 641. Independently of this phosphorylation event and corepressor release, PC4 is recruited by Sp1 and plays an important role as a linker and/or in the formation/assembly of the general transcriptional machinery (1, 3).
The LHR is a G-coupled seven-transmembrane receptor, which is expressed primarily in the gonads, where it mediates luteinizing hormone signals that regulate cyclic ovarian changes or testicular function. Our earlier findings demonstrated that LHR gene expression at the transcriptional level is regulated by complex and diverse networks, in which coordination and interactions between these regulatory effectors are essential for silencing/activation of LHR expression (Figure 1). The proximal Sp1 site of the LHR promoter recruits histone deacetylases and the Sin3A corepressor complex that contributes to the silencing of LHR transcriptional expression. Site-specific acetylation/methylation induce cell-specific phosphatase release, which serves as an "on" mechanism for Sp1 phosphorylation by PI3K/PKCζ at Ser641, causing p107 repressor de-recruitment from Sp1 and LHR transcriptional activation. Maximal derepression of the LHR gene is dependent on complete DNA demethylation of the promoter, histone hyperacetylation, and release of repressors (p107 and HDAC/Sin3A), causing recruitment of TFIIB and Pol II and transcriptional activation (1). Our more recent studies demonstrated that Positive Cofactor 4 (PC4) plays an important role in the formation/assembly of the general transcriptional machinery in trichostatin-A (TSA)–mediated LHR transcription (3). The PC4's recruitment by Sp1 is enhanced following TSA treatment and the cofactor acts as a coactivator of Sp1. The coactivator domain of PC4 and DNA-binding domain of Sp1 are essential for PC4's and Sp1's interaction. However, PC4 does not participate in TSA–induced chromatin structural alteration, given that silencing of PC4 does not prevent release of HDAC/Sin3A and phosphatase from Sp1. We demonstrated that these two events are dependent on DNA methylation and chromatin decompactation resulting from histone acetylation at the promoter. Although TFIIB recruitment is largely dependent on PC4, we ruled out TFIIB as direct target to link Sp1/PC4 to the transcriptional machinery. Given that we anticipate direct or indirect interaction with other members of the initiation and or/mediator complex, we are currently investigating PC4's linking function using various strategies. We excluded acetylation of PC4, either basally or induced by TSA, in the transcriptional activation process. However, immunoprecipitation studies demonstrated that TSA specifically induced acetylation of two PC4–interacting proteins (Mr 10 and 15 kDa). We are proceeding to identify the proteins to determine their role in LHR gene transcription.
Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25)
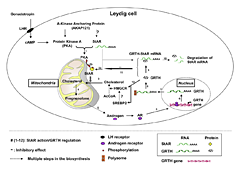
Click image to enlarge.
Figure 2. GRTH–negative autocrine molecular mechanism of regulation of androgen synthesis in Leydig cells
Sequence of events of gonadotropic hormone stimulation of androgen synthesis through PKA–regulated StAR action and GRTH–negative regulation (steps 1–12). Dotted arrows indicate steps (abbreviated) on the biosynthesis and steroidogenesis assessed in this study. ? not determined. Following LH binding to cell-surface luteinizing hormone receptors (LHR) in Leydig cells, cAMP–activated PKA type II in the vicinity of mitochondria phosphorylates StAR. The StAR protein enhances the transfer of cholesterol to the inner mitochondrial membrane, as precursor pregnenolone synthesis enhances androgen production. Via the androgen receptor, androgen activates GRTH gene transcription. GRTH transports its own message from the nucleous to the cytoplam for translation at cytoplasmic sites. GRTH associates with StAR mRNA (StAR mRNA complex) to enhance StAR mRNA degradation (probably through a small–RNA system). The negative regulatory action of GRTH on the fate of StAR mRNA controls androgen homeostasis in Leydig cells. In the absence of GRTH in GRTH knockout, prolonged StAR mRNA half-life and enhanced protein levels facilitate cholesterol accumulation at the inner mitochondria and elevated androgen production upon gonadotropin stimulation (5).
Discovered in our laboratory, GRTH /DDX25 is a testis-specific member of the DEAD-box family of RNA helicases present in Leydig cells (LC) and germ cells. It is a multi-functional protein that is essential for the completion of spermatogenesis. Males lacking GRTH are sterile because round spermatids fail to elongate. Normal basal levels of testosterone in serum and LCs excluded abnormal steroidogenesis as responsible for the arrest of spermiogenesis. Our recent studies revealed that GRTH has an important regulatory role in gonadotropin-induced androgen production by LCs (5). In KO mice, these cells exhibited fewer lipid droplets, swollen mitochondria (site of conversion of cholesterol supplied by lipid droplets, to pregnenolone), and elevated cholesterol content in the inner mitochondrial membrane, which resulted from raised levels of StAR, a protein that transports cholesterol to the inner mitochondrial membrane, and HMGCR, a key enzyme in cholesterol biosynthesis. The half-life of StAR mRNA was significantly higher in the KO mice than in the WT, and we observed association of StAR mRNA with GRTH protein in WT mice. Upon hCG stimulation, both in vivo and in vitro, we observed a major elevation in testosterone levels in KO mice compared with WT that resulted from the availability in KO mice of basal accumulated cholesterol as a substrate of P450scc for pregnenolone production and other distal enzymes of androgen pathway. The finding of an inhibitory action of GRTH associated with gonadotropin-mediated steroidogenesis provides insights into a novel negative autocrine molecular control mechanism of this helicase in the the regulation of steroid production in the male (Figure 2).
GRTH participates in the export of specific mRNAs that are relevant to spermatogenesis from nuclear to cytoplasmic sites of germ cells, including the chromatoid body (CB) of spermatids (storage/processing site) and polyribosomes, where GRTH participates in the translation of relevant messages. In recent studies, we demonstrated that, in addition to being involved in the export of mRNAs of spermatogenic genes, as previously reported, GRTH is involved in the export of its own message from the nucleus to cytoplasmic sites. Association of GRTH mRNA with GRTH protein was observed in testis extracts of WT mice. Blockade of nuclear export with the inhibitor leptomicin b caused significant nuclear accumulation of GRTH mRNA, with major decrease in its cytoplasmic level and its exclusion from the CB. Our studies thus demonstrated the essential participation of the GRTH in export/transport to the CB and other cytoplasmic sites (2).
Prolactin receptor (PRLR)
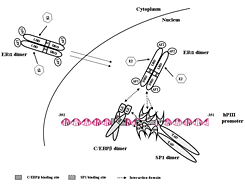
(click image to enlarge)
Figure 3. Model of estradiol (E)-estrogen receptor alpha (ERalpha)-Sp1-CEBPbeta complex formation and binding to the hPIII promoter of the human prolactin receptor gene
Each transcription factor is constitutively present as homodimer. Arrows indicate the interaction at specific binding domains between the proteins through the basic region (BR) and leucine zipper (LZ) of CEBPbeta, zinc finger motifs (ZF1, 2, 3) of Sp1, and DNA–binding domain of ERalpha. The complex is formed by association of the DNA–binding domain of ERα (induced by estradiol) with the ZF motif of Sp1 bound to its consensus element at the promoter (constitutively), followed by recruitment of C/EBPbeta through its LZ. E2 promotes C/EBPbeta binding to its element at the promoter. Interaction between C/EBPbeta and Sp1 stabilizes the complex. This E2–inducible complex is essential for PRLR receptor gene transcription (4).
The prolactin receptor is a member of the lactogen/cytokine receptor family, which mediates the diverse cellular actions of prolactin in several target tissues. Prolactin plays a major role in the proliferation and differentiation of breast epithelium and is essential for the stimulation and maintenance of lactation. The receptor has been implicated also in the development of breast cancer. In humans hPRLR expression is controlled at the transcriptional level by several promoters that were defined and characterized in our laboratory: one generic, also present in rat and mouse (PIII), and five human-specific (hE1N1–hE1N5). The transcription of PRLR in breast cancer cells by the preferentially utilized promoter PIII, which lacks an estrogen-responsive element, is directed by E2/ERalpha through complex formation with Sp1 and C/EBPbeta, which associate with cognate elements and cause TFIIB and Pol II recruitment. BRET revealed ERalpha constitutive homodimers. By stabilizing the dimer, E2 enhances E2/ERalpha-dimer interaction with Sp1 and C/EBPbeta. Chromatin immunoprecipitation and small interfering RNA knockdown of members of the complex in breast cancer cells (ERalpha+) demonstrated endogenous recruitment of components of the complex onto the PIII promoter of the hPRLR gene. Sp1 is the preferred transfactor for the recruitment of ERalpha to the complex that facilitates the C/EBPbeta association. We observed E2/ERalpha–induced hPRLR transcription in ERalpha–negative cells. Recruitment of ERalpha or C/EBPbeta, but not Sp1, was inducible by E2. Cells depleted of C/EBPbeta showed the recruitment of Sp1 and ERalpha. Only Sp1 was associated with PIII when ERalpha was knocked down. The ERalpha-Sp1-C/EBPbeta complex was formed by the association of the DNA-binding domain of ERalpha with zinc fingers (ZF) motifs of Sp1, followed by recruitment of C/EBPbeta through its LeuZipper (LZ). Interactions between ZF of Sp1 and LZ of C/EBPbeta stabilizes the ERalpha-Sp1-C/EBPbeta complex. Our studies demonstrated that the enhanced complex formation of ERalpha dimer with Sp1 and C/EBPbeta dimers at the PIII promoter by E2 plays an essential role in the transcriptional activation of the hPRLR gene in breast cancer cells (4).
Publications
- Dufau ML, Liao M, Zhang Y. Participation of signaling pathways in the derepression of luteinizing hormone receptor transcription. Mol Cell Endocrinol 2010;314:221-227.
- Sato H, Tsai-Morris CH, Dufau ML. Relevance of gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) in the structural integrity of the chromatoid body. Biochim Biophys Acta 2010;1803:534-543.
- Liao M, Zhang Y, Kang JH, Dufau ML. Coactivator function of positive cofactor 4 (PC4) in Sp1-directed luteinizing hormone receptor (LHR) gene transcription. J Biol Chem 2011;286:7681-7691.
- Kang JH, Tsai Morris CH, Dufau ML. Complex formation and interactions between transcription factors essential for human prolactin receptor gene transcription. Mol Cell Biol 2011;31:3208-3222.
- Fukushima M, Villar J, Tsai-Morris CH, Dufau ML. Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) a negative regulator of LH/hCG induced steroidogenesis in Leydig cells. A central role of steroidogenic acute regularory protein (StAR). J Biol Chem 2011;286:29932-29940.
Collaborators
- Sergio A. Hassan, PhD, Center for Molecular Modeling, CIT, NIH, Bethesda, MD
- James Pickel, PhD, Laboratory of Genetics, Transgene Core, NIMH, Bethesda, MD
Contact
For more information, email dufau@helix.nih.gov.

