You are here: Home > Section on Cellular Signaling
Signaling and Secretion in Neuroendocrine Cells

- Stanko S. Stojilkovic, PhD, Head, Section on Cellular Signaling
- Melanija Tomić, PhD, Staff Scientist
- Ivana Bjelobaba, PhD, Visiting Fellow
- Claudio E. Coddou Alvarez, PhD, Visiting Fellow
- Marek Kucka, PhD, Visiting Fellow
- Ellias Leiva-Salcedo, PhD, Visiting Fellow
- Ivan Milenkovic, PhD, Special Volunteer
- Paula Bargi de Souza, MS, Special Volunteer
- Vendula Tvrdonova, MS, Special Volunteer
Using multidisciplinary and collaborative approaches, the Section investigates signaling pathways at the cellular and molecular levels, determines the manner in which hormones and neurotransmitters utilize calcium as an intracellular messenger, and characterizes channels involved in electrical activity and calcium signaling in hypothalamic, pituitary, and other cell types. The Section has investigated cellular signaling cascades and secretion in neuroendocrine cells, with special emphasis on the interactions between plasma membrane electrical events and receptor-controlled pathways. Currently, we are investigating how the structural features of pituitary channels relate to the channels' functions and how plasma membrane receptors and the intracellular signaling milieu affect channel activity. For this purpose, we characterize native and recombinant channels and receptors that were cloned from the pituitary gland. We also analyze the relevance of these channels and pathways to calcium-dependent cellular processes.
Electrophysiological properties of pituitary cells
The membrane potential of isolated pituitary cells in vitro is not stable but oscillates between resting potentials of −65 to −50 mV, reflecting the balance between the activity of depolarizing and hyperpolarizing channels. Channels contributing to the spike depolarization and repolarization in spontaneously firing pituitary cells have been identified. In contrast, very little is known about channels controlling resting membrane potential and initiation of firing of action potentials. Last year, we focused on the role of two non-selective cation channels in electrical activity, calcium signaling, and hormone secretion: hyperpolarization-activated and cyclic nucleotide–gated (HCN) channels and "classic" transient receptor potential channels (TRPC).
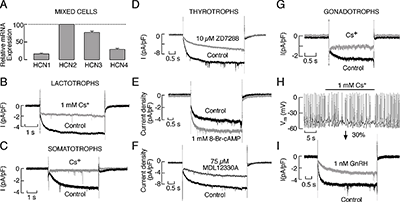
Click image to enlarge.
Figure 1. Expression of HCN channels in pituitary cells
A: Quantitative RT–PCR analysis of the HCN mRNA transcript expression in cultured anterior pituitary cells. B and C: Electrophysiological and pharmacological characterisation of HCN channels in lactotrophs (B) and somatotrophs (C). D–F: Characterisation of the Ih current in rat pituitary thyrotrophs. D: Inhibition of the Ih current by ZD7288. E: Stimulation of Ih by 8-Br–cAMP. F: Inhibition of Ih by MDL12230A, an AC inhibitor. G–I: Properties of the HCN channels in pituitary gonadotrophs. G: Blockade of the Ih current by the addition of 1 mM cesium to the extracellular solution. H: Effects of Ih blockade on the frequency of spontaneous firing of APs in gonadotrophs. I: Inhibition of Ih by GnRH. In B, C, D, E, F, G and I, representative traces of the whole-cell current response to a hyperpolarizing voltage step to −120 mV from a holding potential of −40 mV are shown. Derived from (1).
We studied the role of HCN channels in cultured somatotrophs, gonadotrophs, lactotrophs, and thyrotrophs. The biophysical properties of these channels were systematically investigated in these cell types, including the slow kinetics and voltage dependence of their activation and the lack of inactivation. We also examined the pharmacological properties of the channels. Because there is not a common Gs-coupled receptor among these cells, we used forskolin, an activator of adenylyl cyclase, and cell-permeable 8-Br-cAMP and 8-Br-cGMP to activate the channels. Similarly, we down-regulated basal cAMP production and cAMP–dependent channel activity by inhibiting adenylyl cyclase. We also used pharmacological manipulations to alter the status of phospholipase C activity and phosphoinositide levels. Quantitative RT–PCR analysis showed higher level of expression of mRNA transcripts for HCN2 and HCN3 subunits and lower expression of HCN1 and HCN4 subunits in these cells (Figure 1). Western immunoblot analysis of lysates from normal and GH3 immortalized pituitary cells revealed bands with appropriate molecular weights for HCN2, HCN3, and HCN4. Electrophysiological experiments showed the presence of a slowly developing hyperpolarization-activated inward current, which was blocked by cesium and ZD7288, in gonadotrophs, thyrotrophs, somatotrophs and a fraction of lactotrophs, as well as in other unidentified pituitary cell types (Figure 1). Stimulation of adenylyl cyclase and addition of 8-Br–cAMP enhanced the current and depolarized the cell membrane whereas 8-Br–cGMP did not alter the current and hyperpolarized the cell membrane. Both inhibition of basal adenylyl cyclase activity and stimulation of phospholipase C signaling pathway inhibited this current. Inhibition of HCN channels affected the frequency of firing but did not abolish spontaneous electrical activity. The experiments indicate that cAMP and cGMP have opposite effects on the excitability of endocrine pituitary cells, that basal cAMP production in cultured cells is sufficient to integrate the majority of HCN channels in electrical activity, and that depletion of PIP2 caused by activation of phospholipase C silences them (1, 2).
In cultured lactotrophs and immortalized GH3 cells, replacement of extracellular sodium with large organic cations, but not blockade of voltage-gated sodium influx, led to an instantaneous hyperpolarization of cell membranes that was associated with a cessation of spontaneous firing. When cells were clamped at −50 mV, which was close to the resting membrane potential in these cells, replacement of bath sodium with organic cations resulted in an outward-like current, reflecting an inhibition of the inward holding membrane current and indicating loss of a background-depolarizing conductance. Quantitative RT–PCR analysis revealed high expression of mRNA transcripts for TRPC1 and much lower expression of TRPC6 in both lactotrophs and GH3 cells. Very low expression of TRPC3, TRPC4, and TRPC5 mRNA transcripts was also detected in pituitary but not GH3 cells. The relatively selective blockers of TRPC channels 2-APB and SKF-96365 inhibited electrical activity, calcium influx, and prolactin release in a concentration-dependent manner. Gadolinium, a common calcium channel blocker, and flufenamic acid, an inhibitor of non-selective cation channels, also inhibited electrical activity, calcium influx, and prolactin release. The results indicate that nonselective cation channels, presumably belonging to the TRPC family, contribute to the background depolarizing conductance and firing of action potentials with consequent contribution to calcium influx and hormone release in lactotrophs and GH3 cells (2).
Gating properties and function of pituitary purinergic receptor channels
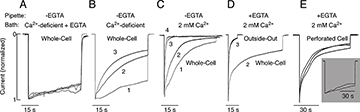
Click image to enlarge.
Figure 2. Dependence of P2X2aR desensitization kinetics on bath and intrapipette calcium concentration
All experiments were performed in HEK293 cells. A–C: Effects of variable bath calcium concentrations on P2X2aR desensitization. Cells were bathed in calcium-deficient Krebs Ringer buffer containing 0.5 mM EGTA (A), calcium-deficient buffer (B), and 2 mM calcium-containing buffer (C). In all experiments, the intrapipette buffer did not contain EGTA. D and E: Dependence of the rate of P2X2aR desensitization on the mode of recording. Cells were bathed in Krebs Ringer buffer and stimulated with 100 µM ATP. D: Whole-cell (second agonist application) vs. outside-out macropatch (third agonist application) recording. E: Perforated cell recording. Main panel: Cells were bathed in 2 mM calcium-containing buffer. Inset: Cells were bathed in calcium-deficient medium. Images derived from (4).
We cloned five ATP–gated P2X receptor channels (P2XRs) from the pituitary gland: P2X2R, P2X3R, P2X4R, P2X6R, and P2X7R (3). Our ongoing work, done in part in collaboration with Arthur Sherman, focuses on P2X2R and P2X7R, which are expressed in a large variety of cells. The channels exhibit two opposite activation-dependent changes—pore dilation and pore closing (desensitization)—through a process that is incompletely understood. To address this question and to clarify the roles of calcium and the C-terminal domain in gating, we combined biophysical and mathematical approaches, using two splice forms of receptors: the full size form (P2X2aR) and the shorter form lacking 69 residues in the C-terminal domain (P2X2bR). Both receptors developed conductivity for N-methyl-D-glucamine within 2–6 s of ATP application. However, pore dilation was accompanied by a decrease rather than an increase in the total conductance, which temporally coincided with rapid and partial desensitization. During sustained agonist application, receptors continued to desensitize in calcium-independent and calcium-dependent modes. Calcium-independent desensitization was more pronounced in P2X2bR and calcium-dependent desensitization in P2X2aR. In whole-cell recording, we also observed use-dependent facilitation of desensitization of both receptors (Figure 2). Such behavior was accounted for by a 16-state Markov kinetic model describing ATP binding/unbinding and activation/desensitization. The model assumes that naive receptors open when two to three ATP molecules bind and that the receptors undergo calcium-independent desensitization, which causes a decrease in the total conductance, or pore dilation, leading to a shift in the reversal potential. In calcium-containing media, receptor desensitization is facilitated and the use-dependent desensitization can be modeled by a calcium-dependent toggle switch. The experiments and the model together provide a rationale for the lack of sustained current growth in dilating P2X2Rs and show that receptors in the dilated state can also desensitize in the presence of calcium (4).
P2X7R is a member of the ATP–gated ion channel family that exhibits distinct electrophysiological and pharmacological properties, including low sensitivity to ATP, lack of desensitization, a sustained current growth during prolonged receptor stimulation accompanied by development of permeability to large organic cations, and the coupling of receptor activation to cell blebbing and death. The uniquely long C-terminus of P2X7R accounts for many of these receptor-specific functions (3). The aim of our recent study, conducted in collaboration with Hana Zemkova, was to understand the role of conserved ectodomain cysteine residues in P2X7R function. Single- and double-point threonine mutants of C119–C168, C129–C152, C135–C162, C216–C226, and C260–C269 cysteine pairs were expressed in HEK293 cells and studied using whole-cell current recording. All mutants other than C119T-P2X7R responded to initial and subsequent application of 300 microM BzATP and ATP with small amplitude monophasic currents or were practically non-functional. The mutagenesis-induced loss of function was attributable to decreased cell-surface receptor expression, as revealed by assessing levels of biotinylated mutants. Coexpression of all double mutants with the wild-type receptor had a transient or, in the case of the C119T/C168T double mutant, sustained inhibitory effect on receptor trafficking. The C119T-P2X7R mutant was expressed on the plasma membrane and was fully functional with a slight decrease in the sensitivity for BzATP, indicating that interaction of liberated Cys168 with another residue rescues the trafficking of receptor. Thus, in contrast to other P2XRs, all disulfide bonds of P2X7R are individually essential for proper receptor trafficking.
Expression and roles of pannexins in ATP release in the pituitary gland
Pannexins are a newly discovered three-member family of proteins, expressed in the brain and peripheral tissues, that belong to the superfamily of gap junction proteins. However, in mammals pannexins do not form gap junctions, and their expression and function in the pituitary gland have not been studied. The last year, we reported that Pannexin 1 (Panx1) is expressed in the pituitary gland and provides a pathway for delivery of ATP release (Zemkova et al. Endocrinology 2011;152:2342). More recent experiments revealed that, in addition to the full size isoform of Panx1, hereafter referred to as Panx1a, pituitary cells also express two novel splice isoforms, termed Panx1c and Panx1d, whose formation reflects the existence of alternative splicing sites in exons 2 and 4. Panx1c lacks the Phe108-Gln180 sequence, and P2X1d the Val307-Cys426 C-terminal sequence. Confocal microscopy and biotin labeling revealed that Panx1a is expressed in the plasma membrane, whereas Panx1c and Panx1d show the cytoplasmic localization when expressed as homomeric proteins. In co-expression studies, we further investigated the interactions of Panx1a with its two splice forms, the effect of expression of these short splice isoforms on the ATP release functions of full-size Panx1a channels, and their association with P2XRs. The three Panx1 isoforms and Panx2 form homomeric and heteromeric complexes in any combination. The splice forms can also physically associate with ATP–gated P2X2, P2X3, P2X4, and P2X7 receptor channels. Panx1a–mediated ATP release in AtT-20 immortalized pituitary cells is attenuated when co-expressed with Panx1c or Panx1d. The results suggest that Panx1c and Panx1d may serve as dominant-negative effectors to modulate the functions of Panx1a through the formation of heteromeric channels. The complex patterns of Panx1 expression and association could also define the P2X–dependent roles of these channels in cell types co-expressing both proteins (5).
Publications
- Kretschmannova K, Kucka M, Gonzalez-Iglesias AE, Stojilkovic SS. The expression and role of hyperpolarization-activated and cyclic nucleotide-gated channels in endocrine anterior pituitary cells. Mol Endocrinol 2012;26:153-164.
- Stojilkovic SS, Kretschmannova K, Tomic M, Stratakis CA. Dependence of the excitability of pituitary cells on cyclic nucleotides. J Neuroendocrinol 2012;24:1183-1200.
- Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev 2011;63:641-683.
- Khadra A, Yan Z, Coddou C, Tomic M, Sherman A, Stojilkovic SS. Gating properties of the P2X2a and P2X2b receptor channels: experiments and mathematical modeling. J Gen Physiol 2012;139:333-348.
- Li S, Tomic M, Stojilkovic SS. Characterization of novel pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channels. Gen Comp Endocrinol 2011;174:202-210.
Collaborators
- J. Pablo Huidobro-Toro, PhD, Catholic University, Santiago, Chile
- Michael J. Iadarola, PhD, Laboratory of Sensory Biology, NIDCR, Bethesda, MD
- Anmar Khadra, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
- Arthur Sherman, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
- Constantine Stratakis, MD, D(med)Sci, Program in Developmental Endocrinology and Genetics, NICHD, Bethesda, MD
- Hana Zemkova, PhD, Institute of Physiology, Academy of Science, Prague, Czech Republic
Contact
For more information, email stankos@helix.nih.gov or visit neuroscience.nih.gov/Faculty/Profile/stanko-stojilkovic.aspx.

