You are here: Home > Section on Metabolic Regulation
Signal Transduction in Synaptic Transmission and Plasticity

- Kuo-Ping Huang, PhD, Head, Section on Metabolic Regulation
- Freesia L Huang, PhD, Staff Scientist
- Binukumar Balachandran, PhD, Visiting Fellow
- Yihsin Chen, BS, Postbaccalaureate Intramural Research Training Award Fellow
We investigate the signal transduction mechanisms involved in synaptic transmission and plasticity. Studies of these neural processes are essential to understanding the complex problems related to cognition and behavioral abnormalities. We currently focus on genetically modified mice with deletion of the gene encoding neurogranin (Ng), which is specifically expressed in the brain. Ng knockout mice exhibit cognitive deficits and several behavioral abnormalities, including hyperactivity, impulsivity, social withdrawal, and attention deficits. Ng is normally expressed at a high level in subsets of neurons in the forebrain. The protein has been implicated in the regulation of Ca2+- and Ca2+/calmodulin (CaM)–dependent cellular processes important for the enhancement of synaptic transmission and plasticity. In humans, mutation of the Ng gene has been linked to behavioral abnormalities and cognitive deficits, including schizophrenia, bipolar disorder, and attention deficit and hyperactivity disorder. In the neuronal soma and dendrites Ng levels are very high, and Ng sequesters apoCaM at basal physiological Ca2+ levels. Upon synaptic stimulation, the influxed Ca2+ displaces Ng from the Ng/apoCaM complex to form Ca2+/CaM and free Ng. Ng then becomes a substrate for protein kinase C. The resulting phosphorylated Ng no longer binds to CaM and thus prolongs the stimulation of CaM-dependent enzymes involved in synaptic responses. In addition, free Ng is readily oxidized by oxidants generated during synaptic stimulation, and the resulting oxidized Ng also exhibits a low affinity for CaM, with a functional consequence on synaptic responses similar to that of the phosphorylated Ng. The multifarious regulation of Ng by Ca2+, phosphorylation, and oxidation indicates that this protein as a critical player in the modulation of synaptic plasticity. The buffering of CaM by Ng serves as a mechanism to regulate neuronal free Ca2+ and Ca2+/CaM concentrations. The aim of this project is to delineate the fine-tuning regulatory functions of Ng in neuronal signaling and to design therapeutic approaches to treat cognitive deficits and behavioral disturbances in humans suffering from mutations in Ng.
Treatment of neurogranin knockout mice with methylphenidate (Ritalin) improves their cognition and behavioral abnormalities.
Click image to enlarge.
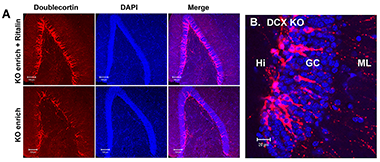
Figure 1. Treatment of NgKO mice kept in enriched environment with Ritalin raised the number of doublecortin (DCX)-positive neuronal precursor cells.
A: Immunohistochemical staining of hippocampal sections derived from Ritalin-treated and untreated NgKO mice with antibody against DCX. The DCX–positive cells were largely congregated at the subgranular zone of dentate gyrus. B: Higher magnification of the DCX–positive cells showed the migration of precursor cells from subganular zone within hilus (Hi) into the granule cell layer (GC), with dendrites extending to the molecular layer (ML).
Deletion of Ng in mice (NgKO) caused down-regulation of Ca2+-mediated signaling, deficits in cognitive functions, and high frequency stimulation (HFS)–induced long-term potentiation (LTP) in hippocampal slices. Further characterization revealed that the animals exhibited additional behavioral abnormalities, including hyperactivity, impulsivity, and social withdrawal. The behavioral phenotypes likely result from disruption of Ng-regulated signaling. Environmental enrichment (EE) alone failed to improve the cognitive function of these mutant mice. We therefore treated the NgKO mice, kept under EE, with methylphenidate (MPH, Ritalin), a psychostimulant drug known to raise extracellular dopamine. Four groups of animals, including control and drug-treated wild-type and NgKO mice, were injected with MPH (10 mg/kg/day, i.p.) for three weeks and then subjected to behavioral tests during the following two weeks. Cognitive functions of the drug-treated NgKO mice improved, as evidenced by a reduction in the latency time to locate the hidden platform in the water maze and an increase in the freezing time after contextual fear conditioning. MPH also reduced the hyperactivity of NgKO in the open field and increased the immobility time in the forced-swim chamber. The drug-treated mutant mice exhibited improvement in their social interaction with other mice and in recognition of novel mice. The drug treatment, however, had only a marginal effect on the performance of wild-type mice. Measurement of the HFS–induced LTP (one train of 100 Hz for 1s) in the hippocampal CA1 region in vitro showed a positive effect of the drug on the NgKO hippocampus. At the cellular level, treatment of NgKO with MPH increased glial fibrillary acidic protein (GFAP)–positive astrocytes in the hippocampus, especially prominently in the hilus of dentate gyrus and stratum radiatum of the CA1 region. In the hilus, a large number of astrocytes accumulated at the subgranular layer, which is known to harbor subpopulations of neural stem cells. Indeed, the drug-treated NgKO exhibited an increase in the number of proliferative Ki-67-positive cells and doublecortin-positive neuronal precursor cells, an indication of increasing neurogenesis (Figure 1). This structural remodeling may underlie drug-mediated neurobehavioral responses. The results indicate that MPH, a drug commonly used for the treatment of attention-deficit hyperactivity disorder (ADHD), can exert beneficial effects on the NgKO mouse as it does for the human patients. The studies also suggest that NgKO mice may be useful for the development of new treatment strategies for certain behavioral deficits related to ADHD, schizophrenia, and bipolar disorder.
Enhancement of hippocampal synaptic plasticity in neurogranin-knockout mice by phorbol ester
Click image to enlarge.
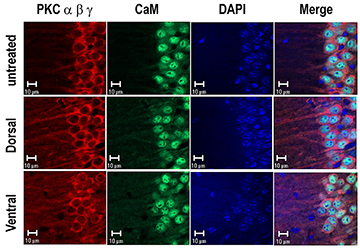
Figure 2. Treatment of hippocampal slices with PKC-activating phorbol ester induced translocation of PKCs.
Treatment of hippocampal slices with phorbol ester caused an increase in the amplitude of population spike of both dorsal and ventral slices but only an increase in the slope of excitatory postsynaptic potential of the former. In spite of these differences in the electrophysiological responses, phorbol ester caused translocation of PKC α, β, and γ from soma to dendrites in both hippocampal segments.
One of the most prominent roles of Ng is the enhancement of the NMDA receptor–mediated Ca2+ transients. A rise in intracellular Ca2+ triggers stimulation of Ca2+- and Ca2+/CaM–activated enzymes and their downstream targets to promote synaptic responses and plasticity. Thus, stimulation of downstream signaling components after Ca2+ influx or increase of presynaptic transmitter release may rescue the deficits of NgKO mice. In neurons, phorbol ester is known to enhance transmitter release and facilitate postsynaptic responses by PKC–mediated phosphorylation of AMPA receptors. Short-term treatment (20 min) of hippocampal slices from NgKO mice with the PKC–activating phorbol ester phorbol 12,13-dibutylate (PDBu) or phorbol 12,13-diacetate (PDAc) caused persistent synaptic facilitation in the hippocampal CA1 region, which lasted for several hours. The treatments also promoted the redistribution of PKCs from soma to dendrites (Figure 2). The phorbol ester–mediated effects were most pronounced among those tissue slices from the dorsal hippocampus that exhibited positive responses in the field excitatory postsynaptic potential (fEPSP) and the amplitude of population spike (POPS). In contrast, phorbol ester only enhanced the amplitude of POPS and had no significant effect on the fEPSP of tissue slices from ventral hippocampus. For dorsal hippocampal slices, the initial rate of the phorbol ester–induced stimulation in fEPSP was dose-dependent and was inhibited by the PKC inhibitor chelerythrine but not by the CaMKII inhibitor KN93, the MEK inhibitor U0126, the protein synthesis inhibitor anisomycin, or the NMDA–receptor antagonist APV. Following maximal stimulation by phorbol ester, application of theta-burst electrical stimulation (TBS) caused no additional response. However, prior stimulation with TBS followed by phorbol ester induced additional potentiation of fEPSP, suggesting that the phorbol ester–mediated responses also overlap with those caused by electrical stimulation, which triggers the activation of postsynaptic NMDA receptors and subsequent stimulation of multiple kinases including PKC. It is intriguing that, for tissue slices from the ventral hippocampus, application of phorbol ester following TBS induced depotentiation. The findings clearly distinguish the phorbol ester–mediated physiological responses of the dorsal from those of the ventral hippocampus. The positive responses of dorsal hippocampus of NgKO mice to phorbol ester–mediated long-term facilitation suggest that treatment of these animals with an analog of the PKC activator (diacylglycerol) may improve the synaptic efficacy of this area, which is thought to be associated with cognitive functions.
Publications
- HHuang K-P, Huang FL, Shetty PK. Stimulation-mediated translocation of calmodulin and neurogranin from soma to dendrites of mouse hippocampal CA1 pyramidal neurons. Neuroscience 2011;178:1-12.
- Huang K-P, Huang FL. Calcium-sensitive translocation of calmodulin and neurogranin between soma and dendrites of mouse hippocampal CA1 neurons. ACS Chem Neurosci 2011;2:223-230.
- Huang FL, Huang K-P. Methylphenidate improves the behavioral and cognitive deficits of neurogranin knockout mice. Genes Brain Behav 2012;11:794-805.
Contact
For more information, email kphuang@helix.nih.gov or visit neuroscience.nih.gov/Faculty/Profile/kuo-ping-huang.aspx

