You are here: Home > Section on Molecular Signal Transduction
Phosphoinositide Messengers in Cellular Signaling and Trafficking

- Tamás Balla, MD, PhD, Head, Section on Molecular Signal Transduction
- Yeun Ju Kim, PhD, Staff Scientist
- Naveen Bojjireddy, PhD, Postdoctoral Fellow
- Maria-Luisa Guzman-Hernandez, PhD, Postdoctoral Fellow
- Gerald Hammond, PhD, Postdoctoral Fellow
- Marko Jovic, PhD, Postdoctoral Fellow
The Section on Molecular Signal Transduction investigates signal transduction pathways that mediate the actions of hormones, growth factors, and neurotransmitters in mammalian cells, with special emphasis on the role of phosphoinositide-derived messengers. Phosphoinositides constitute a small fraction of the cellular phospholipids but play critical roles in the regulation of many signaling protein complexes that assemble on the surface of cellular membranes. Phosphoinositides regulate protein kinases and GTP–binding proteins as well as membrane transporters, including ion channels, thereby controlling many cellular processes such as proliferation, apoptosis, metabolism, cell migration, and differentiation. We focus on the phosphatidylinositol 4 (PtdIns4)–kinases (PI4Ks), a family of enzymes that catalyze the first committed step in polyphosphoinositide synthesis. Current work aims to: (i) understand the function and regulation of several PI4Ks involved in the control of cellular signaling and trafficking pathways; (ii) find specific inhibitors for the individual PI4Ks; (iii) define the molecular basis of PtdIns4P–regulated pathways through identification of PtdIns4P–interacting molecules; (iv) develop tools to analyze inositol lipid dynamics in live cells; and (v) determine the importance of the lipid-protein interactions in the activation of cellular responses by G protein–coupled receptors and receptor tyrosine kinases.
Analysis of the relationship between PI4P and PI(4,5)P2 and their regulatory functions in the plasma membrane
Polyphosphoinositides (PPIs) are phosphorylated forms of phosphatidylinositol (PtdIns) formed by a variety of PI and PIP kinases in eukaryotic cells. Although present in minute amounts, these phospholipids are of enormous interest because of their pivotal roles in regulating virtually every cellular process within eukaryotic cells. They were first recognized as precursors of important second messengers, generated upon stimulation of certain groups of cell-surface receptors. However, PPIs have proven to be more versatile in that they also regulate ion channels and transporters and control membrane fusion and fission events and hence are master regulators of vesicular transport, secretion, and endocytosis; they also play key roles in lipid transport and disposition.
In a set of studies performed in collaboration with the Irvine group, we studied the role of the plasma membrane phosphoinositides, PI4P and PI(4,5)P2 in cellular regulation. PI4P is a metabolic precursor of PI(4,5)P2 in the plasma membrane, and the two lipids often change in parallel during physiological stimulation of cell surface receptors. To enable us to alter the levels of these two lipids separately, we developed a novel molecular tool, which consisted of a hybrid enzyme formed from a PI(4,5)P2 5-phosphatase and the yeast Sac1 phosphatase that hydrolyses PI4P. The hybrid enzyme, called pseudojanin (PJ), was acutely recruited to the plasma membrane by a drug-induced heterodimerizetion system developed in our laboratory. We generated versions of PJ with defective 5-phosphatase or 4-phosphatase activities (or lacking both activities) and, with these tools, we could selectively manipulate PI4P and PI(4,5)P2 levels in a rapidly controlled manner. The studies showed that cells are able to maintain their PI(4,5)P2 pools at a wide range of PI4P levels provided that the PI4K enzyme(s) that generate PI4P at the plasma membrane are functional. However, PI4P in the plasma membrane turned out to play a very important role in supplying an electrostatic interaction that is required for proper functions of some ion channels and for the membrane targeting of a whole set of peripheral membrane proteins, such as several small GTP–binding proteins. The studies uncovered an important and hitherto unrecognized role of PI4P at the plasma membrane, namely, its ability to fulfill functions that had been traditionally attributed to PI(4,5)P2. It is also notable that differences exist between proteins that selectively require PI(4,5)P2 (such as the cold-sensing menthol channels) and those that can use either lipids to support their functions (such as the heat-sensing vanilloid receptors). The importance of such studies is that they help us better understand how some of the most important regulators of cell proliferation, such as the Ras proteins that are mutated in a significant number of cancers, bind to the plasma membrane, given that membrane binding is critical for the normal and oncogenic activities of these proteins.
Two phosphatidylinositol 4-kinases control lysosomal delivery of the Gaucher disease enzyme β-glucocerebrosidase.
This set of experiments focused on the question of how the beta-glucocerebrosidase enzyme (GBA) reaches the lysosomes. Defects in the function of this enzyme cause Gaucher disease, one of the most common human lysosomal storage diseases. GBA is synthesized in the endoplasmic reticulum (ER) and binds to LIMP2, a receptor protein that carries GBA to the lysosome. In a proteomic analysis, both GBA and LIMP2 were found in a complex with PI4KB, one of the phosphatidylinositol kinase enzymes. Inositol lipids constitute a small fraction of membrane phospholipids but have important regulatory functions. We determined whether PI4P, the lipid product of PI4KB, is important for LIMP2/GBA transport to the lysosome. We found that distinct PI4Ks play important roles at several steps in the trafficking pathway of the LIMP-2/GBA complex. Acute depletion of PI4P in the Golgi caused accumulation of LIMP-2 in this compartment, and PI4KB was found to be responsible for controlling the exit of LIMP-2 from the Golgi. In contrast, depletion of PI4K2A, another PI4K, blocked trafficking at a post-Golgi compartment, leading to accumulation of LIMP-2 in enlarged endosomal vesicles. PI4K2A depletion also caused secretion of missorted GBA into the medium, which was attenuated by limiting LIMP-2/GBA exit from the Golgi using PI4KB inhibitors. The studies identified PI4KB and PI4K2A as important regulators of lysosomal delivery of GBA, revealing a new element of control to sphingolipid homeostasis by phosphoinositides. The importance of these studies is that PI4P and sphingolipids are not only critical for cellular functions, but also for replication of certain viruses within the cell. Several Pharma companies are developing PI4K inhibitors as potential antiviral agents. It is critical that we understand what processes are controlled by these enzymes in the cell to properly evaluate the potential risks and benefits from the use of such inhibitors.
Acute depletion of plasma membrane PI(4,5)P2 impairs specific steps in endocytosis of the G protein–coupled receptor.
In studies performed in collaboration with Péter Várnai's group, we again used our recruitable inositol lipid 5-phosphatase system to study the PI(4,5)P2 requirement of G protein–coupled receptor internalization using AT1 angiotensin, beta-2 adrenergic, and type-2C serotoninergic receptors. We followed the movement of the receptors between the various compartments from the plasma membrane to early endosomes by bioluminescence energy transfer (BRET), using receptors fused to luciferase and YFP targeted to the surface of various organelles. We then followed receptor movement in cells in which PI(4,5)P2 was either left unchanged or depleted by the recruited 5-phosphatase enzyme. The studies showed that ligand-induced interaction of AT1, 5HT2C, and beta2A receptors with beta-arrestin-2 was unaffected by PI(4,5)P2 depletion. However, trafficking of the receptors to Rab5–positive early endosomes was completely abolished in the absence of PI(4,5)P2. Remarkably, removal of the receptors from the plasma membrane was reduced but not eliminated after PI(4,5)P2 depletion and, in this case, the stimulated AT1 receptors clustered along the plasma membrane without entering the cells. The data suggest that, in the absence of PI(4,5)P2, the receptors move into clathrin-coated membrane structures that are not cleaved efficiently and hence cannot reach the early endosomal compartment. The significance of such studies is that G protein–coupled receptors regulate almost every process in the human body and are targeted by many human medicines. Cells regulate the sensitivity of these receptors by the very processes that are the focus of these studies, and a better understanding of the pathways will help us decipher disease mechanisms and design better drugs.
Further characterization of the newly identified ER–derived phosphatidylinositol-synthesizing organelle
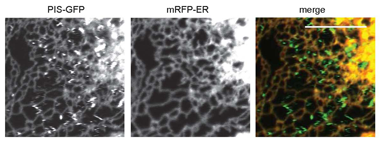
Click image to enlarge.
Figure 1. Distribution of phosphatidylinositol synthase (PIS) in live cells
COS-7 cells were transfected with the PIS enzyme fused to GFP and an ER–targeted mRFP construct. Cells were studied in a Zeiss LSM510 Confocal Microscope. The rapidly moving PIS–GFP objects show up as zig-zags on these pictures, which demonstrate that PIS is present both in the tubular ER and in rapidly moving compartments.
Last year, we reported that the PtdIns–synthesizing enzyme PIS associates with a rapidly moving compartment of the endoplasmic reticulum (ER) that makes ample contacts with various organelles, including the plasma membrane. We showed that the PIS–positive objects originated from the ER and that their generation was abolished by expression of a GTP–locked form of the small GTPase Sar1, a regulator of ER exit sites. Mutations that rendered PIS catalytically incompetent also prevented the objects' movement to the mobile fraction. Density-gradient fractionation showed that PIS activity was associated primarily with light membrane fractions that were separable from the bulk of the ER. Importantly, the two CDP–diacylglycerol (DAG) synthase (CDS) enzymes that provide PIS with its substrate, CDP-DAG, were found in the tubular ER but not in the PIS–positive organelle. We proposed a model in which PtdIns is synthesized in a highly mobile lipid distribution platform and is delivered to other membranes during multiple contacts by yet-to-be-defined lipid transfer mechanisms.
We continued to study this new organelle and investigated whether the endogenous PIS enzyme is also present in this compartment. Unfortunately, no antibodies are available to detect the PIS enzyme by immunofluorescence, so we recently initiated the production of antibodies against this protein. In the meantime, we performed gradient fractionation in untransfected cells and measured PIS activity of the membranes, confirming that PIS was associated with the light fractions in addition to the ER and indicating that the mobile compartment also exists without PIS overexpression. We also found that overexpression of CDS enzyme elevated the expression of PIS, suggesting that the two enzymes are functionally coupled. Lastly, we generated a construct of PIS fused with a biotynilation tag and performed a biotin-streptavidin–based immuno-isolation of PIS to determine which proteins interact with the enzyme. Proteomic analysis of this sample and verification of the validity and functional relevance of these interactions are currently under way.
Publications
- Hammond GR, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, Irvine RF. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science 2012;337:727-730.
- Jović M, Kean MJ, Szentpetery Z, Polevoy G, Gingras AC, Brill JA, Balla T. Two phosphatidylinositol 4-kinases control lysosomal delivery of the Gaucher disease enzyme, β-glucocerebrosidase. Mol Biol Cell 2012;23:1533-1545.
- Tóth DJ, Tóth JT, Gulyás G, Balla A, Balla T, Hunyady L, Várnai P. Acute depletion of plasma membrane phosphatidylinositol 4,5-bisphosphate impairs specific steps in endocytosis of the G-protein-coupled receptor. J Cell Sci 2012;125:2185-2197.
- Altan-Bonnet N, Balla T. Phosphatidylinositol 4-kinases: hostages harnessed to build panviral replication platforms. Trends Biochem Sci 2012;37:293-302.
- Kim YJ, Guzman-Hernandez ML, Balla T. A highly dynamic ER-derived phosphatidylinositol synthesizing organelle supplies phosphoinositides to cellular membranes. Dev Cell 2011;21:813-824.
Collaborators
- Nihal Altan-Bonnet, PhD, Rutgers, The State University of New Jersey, Newak, NJ
- Ralf Bartenschlager, MD, PhD, Universitätsklinikum Heidelberg, Heidelberg, Germany
- Julie A. Brill, PhD, Hospital for Sick Children, Toronto, Canada
- László Hunyady, MD, PhD, Semmelweis University, Faculty of Medicine, Budapest, Hungary
- Robin F. Irvine, PhD, University of Cambridge, Cambridge, UK
- Péter Várnai, MD, PhD, Semmelweis University, Faculty of Medicine, Budapest, Hungary
Contact
For more information, email ballat@mail.nih.gov.

