You are here: Home > Section on Molecular Dysmorphology
Cholesterol Homeostasis and Genetic Syndromes

- Forbes D. Porter, MD, PhD, Head, Section on Molecular Dysmorphology
- Chris A. Wassif, MSc, Technical Specialist
- Celine Cluzeau, PhD, Postdoctoral Fellow
- Stephanie Cologna, PhD, Postdoctoral Fellow
- Antony Cougnoux, PhD, Postdoctoral Fellow
- Kevin Francis, PhD, Postdoctoral Fellow
- Ian M. Williams, PhD, Postdoctoral Fellow
- Nicole M. Yanjanin, CPNP, Nurse Practitioner
- Lee Ann Keener, RN, Research Nurse
- Joanna Cross, BA, Welcome Trust Student
- Emily Leff, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Jackie Picache, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Gian Rodriguez, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Ryan Lee, MD, Special Volunteer
- Elaine Tierney, MD, Special Volunteer
We study the molecular, biochemical, and cellular processes that underlie genetic disorders resulting from impaired cholesterol homeostasis. The disorders include malformation/cognitive impairment syndromes resulting from inborn errors of cholesterol synthesis, neurodegenerative disorders resulting from impaired intracellular cholesterol and lipid transport, and Autistic Spectrum disorder (ASD) associated with low cholesterol. Human malformation syndromes attributable to inborn errors of cholesterol synthesis include Smith-Lemli-Opitz syndrome (SLOS), lathosterolosis, desmosterolosis, X-linked dominant chondrodysplasia punctata type 2 (CDPX2), and the CHILD syndrome. Neimann-Pick disease type C (NPC) results from impaired intracellular transport of cholesterol and lipids, leading to neuronal loss. Our basic research uses mouse models of these genetic disorders to understand the biochemical, molecular, cellular, and developmental processes that underlie the birth defects and clinical problems encountered in affected patients. Our clinical research focuses on translating basic findings to the clinic. Natural history trials of both SLOS and NPC1 are ongoing. Our emphasis on both basic and clinical research allows us to integrate laboratory and clinical data in order to increase our understanding of the pathological mechanisms underlying both SLOS and NPC, with the goal of improving clinical care of these patients. Therapeutic trials have been conducted for both disorders, and we are conducting a Phase I therapeutic trial of 2-hydroxypropyl-B-cyclodextrin for NPC. During the next year we expect that a multicenter, multinational clinical efficacy trial of 2-hydroxypropyl-B-cyclodextrin will be initiated.
Inborn errors of cholesterol synthesis

Figure 1.
Dr. Porter and one of our patients. Neurological exams in children frequently involve "playing" with the child.
SLOS is an autosomal recessive, multiple-malformation syndrome characterized by dysmorphic facial features, cognitive impairment, hypotonia, poor growth, and variable structural anomalies of heart, lungs, brain, limbs, gastrointestinal tract, and genitalia. The SLOS phenotype is extremely variable. At the severe end of the phenotypic spectrum, infants often die as result of multiple major malformations, while mild SLOS combines minor physical malformations with behavioral and learning problems. The syndrome is attributable to an inborn error of cholesterol biosynthesis that blocks the conversion of 7-dehydrocholesterol (7-DHC) to cholesterol.
Our laboratory initially cloned the human 3β-hydroxysterol Δ7-reductase gene (DHCR7) and demonstrated mutations of the gene in SLOS patients. Together with others, we have so far identified over 100 mutations of DHCR7. We also used gene targeting in murine embryonic stem cells to produce multiple SLOS mouse models, including a null deletion and a hypomorphic point mutation. Mouse pups homozygous for the null mutation (Dhcr7Δ3-5/Δ3-5) exhibit variable craniofacial anomalies, are growth-retarded, feed poorly, appear weak, and die during the first day of life because they fail to feed. Thus, we were not able use them to study postnatal brain development, myelination, or behavior or to test therapeutic interventions. For this reason, we developed a mis-sense allele (Dhcr7T93M). The T93M mutation is the second most common mutation found in SLOS patients. Dhcr7T93M/T93M and Dhcr7T93M/Δ3-5 mice are viable and demonstrate SLOS with a gradient of biochemical severity (Dhcr7Δ3-5/Δ3-5>Dhcr7T93M/Δ3-5>Dhcr7T93M/T93M). We used Dhcr7T93M/Δ3-5 mice to test the efficacy of therapeutic interventions on tissue sterol profiles. As expected, dietary cholesterol therapy improved the sterol composition in peripheral tissues but not in the central nervous system. Treatment of mice with simvastatin improved the biochemical defect in both peripheral and central nervous system tissue, suggesting that simvastatin therapy may be used to treat some of the behavioral and learning problems in children with SLOS. Most recently, we developed a zebrafish model for SLOS that will allow us to study the impact of aberrant cholesterol synthesis on behavior. In addition, we developed and are characterizing SLOS and lathosterolosis induced pleuripotent stem cell lines.
As part of our clinical studies on SLOS, we identified a novel oxysterol, 27-hydroxy-7-dehydrocholesterol (27-7DHC), derived from 7-DHC in SLOS patients. We therefore investigated whether 27-7DHC contributes to the pathology of SLOS and found a strong negative correlation between plasma 27-7DHC and cholesterol levels in these patients. In addition, previous work showed that low cholesterol levels impair hedgehog signaling. Therefore, we hypothesized that increased 27-7DHC levels would have detrimental effects during development in response to suppression of cholesterol levels. To test our hypothesis, we produced SLOS mice (Dhcr7Δ3-5/Δ3-5) expressing a CYP27 (sterol 27-hydroxylase) transgene. CYP27Tg mice display increased CYP27 expression and elevated 27-hydroxycholesterol levels but normal cholesterol levels. While Dhcr7Δ3-5/Δ3-5 mice are growth-retarded, exhibit a low incidence of cleft palate (9%), and die during the first day of life, Dhcr7Δ3-5/Δ3-5:CYP27Tg embryos are stillborn and have multiple malformations, including growth retardation, micrognathia, cleft palate (77%), lingual and dental hypoplasia, ankyloglossia, umbilical hernia, cardiac defects, cloacae, curled tails, and limb defects. We observed autopod defects (polydactyly, syndactyly, and oligodactyly) in 77% of the mice. Consistent with our hypothesis, sterol levels were halved in the liver and 20-fold lower in the brain tissue of Dhcr7Δ3-5/Δ3-5:CYP27Tg than in Dhcr7Δ3-5/Δ3-5 embryos. The fact that 27-7DHC plays a role in SLOS may explain some of the phenotypic variability and may lead to development of a therapeutic intervention. The project is a good example of the benefits of integrating clinical and basic science to both understand the pathology of SLOS and develop potential therapeutic interventions.
To gain insight into pathophysiological processes contributing to SLOS, we completed a series of proteomic experiments to identify proteins that are differentially expressed in the cortex of Dhcr7Δ3-5/Δ3-5 embryos. Functional analysis demonstrated alternations of the Rho/Rac-LIMK-Cofilin pathway that result in altered dendrite and axon formation. The defect in neuronal development may contribute to the cognitive deficits found in SLOS.
We are involved in an ongoing Natural History trial as part of a NICHD/ORD–supported Rare Disease Research Consortium. Given that SLOS patients have a cholesterol deficiency, they may be treated with dietary cholesterol supplementation. To date, we have evaluated over 75 SLOS patients and continue to follow many of them in a longitudinal natural history study. We recently completed a randomized, placebo-controlled, double-blinded cross-over trial to study the safety and efficacy of simvastatin therapy in SLOS. As part of a Bench-to-Bedside proposal, we continue, in collaboration with Fran Platt’s laboratory, to study impaired cholesterol and glycosphingolipid transport in SLOS and to investigate novel therapeutic interventions.
One reason for studying rare genetic disorders is to gain insight into more common disorders. Most patients with SLOS exhibit autistic characteristics. We are currently collaborating with other NIH and extramural groups to evaluate this further.
Lathosterolosis, desmosterolosis, and HEM dysplasia
Lathosterol 5-desaturase catalyzes the conversion of lathosterol to 7-dehydrocholesterol, representing the enzymatic step immediately preceding the defect in SLOS. Thus, to gain a deeper understanding of the roles of reduced cholesterol versus elevated 7-dehydrocholesterol in SLOS, we disrupted the mouse lathosterol 5-desaturase gene (Sc5d) by using targeted homologous recombination in embryonic stem cells. Sc5d−/− pups are stillborn, present with micrognathia and cleft palate, and exhibit limb-patterning defects. Many of the malformations in the mutant mice resemble malformations in SLOS and are consistent with impaired hedgehog signaling during development. Biochemically, the mice exhibit markedly elevated lathosterol levels and reduced cholesterol levels in serum and tissue.
A goal of producing a lathosterolosis mouse model was to gain phenotypic insight for the purpose of identifying a corresponding human malformation syndrome. We identified a human infant patient with lathosterolosis, a malformation syndrome not previously described in humans. Biochemically, fibroblasts from the patient show reduced cholesterol and elevated lathosterol levels. Mutation analysis showed that the patient is homozygous for a single A→C nucleotide change at position 137 in SC5D, resulting in a mutant enzyme in which the amino acid serine is substituted for tyrosine at position 46. Both parents are heterozygous for the mutation. The infant’s phenotype resembled severe SLOS. Malformations found in both the human patient and the mouse model include growth failure, abnormal nasal structure, abnormal palate, micrognathia, and postaxial polydactyly. A unique feature of lathosterolosis is the clinical finding of mucolipidosis in the affected infant, which is not reported in SLOS and may help distinguish SLOS clinically from lathosterolosis. Lathosterolosis, which is a lysosomal storage disorder, may be replicated in embryonic fibroblasts from the Sc5d–mutant mouse model. To distinguish pathological changes attributable to reduced cholesterol in lathosterolosis from those that are a consequence of elevated 7-DHC in SLOS, we are comparing proteomic changes in the Sc5d–mutant mouse model with those in the SLOS mouse model.
Desmosterolosis is another inborn error of cholesterol synthesis that resembles SLOS. It results from a mutation in the 3β-hydroxysterol Δ24-reductase gene (DHCR24). DHCR24 catalyzes the reduction of desmosterol to cholesterol. We disrupted the mouse Dhcr24 gene by using targeted homologous recombination in embryonic stem cells. Surprisingly, although most Dhcr24 mutant mice die at birth, the pups are phenotypically normal.
Others have shown that mutations of the lamin B receptor (LBR) cause HEM (hydrops, ectopic calcification, moth-eaten skeletal) dysplasia in humans and ichthyosis in mice. LBR has both lamin B–binding and sterol Δ14-reductase domains. Although only a minor sterol abnormality has been reported, it was proposed that LBR is the primary sterol Δ14-reductase and that impaired sterol Δ14-reduction underlies HEM dysplasia. However, DHCR14 also encodes a sterol Δ14-reductase. To test the hypothesis that LBR and DHCR14 are redundant sterol Δ14-reductases, we obtained ichthyosis mice (Lbr Sc5d−/−) and disrupted Dhcr14. Dhcr14 Sc5d−/− mice are phenotypically normal. We found no sterol abnormalities in either Lbr Sc5d−/− or Dhcr14 Sc5d−/− tissues at 1 or 21 days of age. We then bred the mice to obtain compound mutant mice. Lbr Sc5d−/−:Dhcr14 Sc5d−/− and Lbr Sc5d−/−:Dhcr14+/− died in utero. Lbr+/−:Dhcr14 Sc5d−/− mice appeared normal at birth but, by 10 days of age, were growth-retarded and neurologically abnormal (with ataxia and tremors) and, consistent with a demyelinating process, evidenced vacuolation and swelling of myelin sheaths in the spinal cord upon pathology evaluation. We observed neither vacuolation nor swelling of myelin sheaths in either Lbr Sc5d−/− or Dhcr14 Sc5d−/− mice. In contrast to Lbr Sc5d−/− mice, Lbr+/−:Dhcr14 Sc5d−/− mice had normal skin and did not display the Pelger-Huët anomaly. Peripheral tissue sterols were normal in all three mutant mice, although we found significantly elevated levels (50% of total sterols) of cholesta-8,14-dien-3β-ol and cholesta-8,14,24-trien-3β-ol in brain tissue from 10-day-old Lbr+/−:Dhcr14 Sc5d−/− mice. In contrast, we observed relatively small transient elevations in Δ14-sterol levels in Lbr Sc5d−/− and Dhcr14Δ4-7/Δ4-7 brain tissue. Our data support the notion that HEM dysplasia and ichthyosis result from impaired lamin B receptor function rather than from impaired sterol Δ14-reduction. Impaired sterol Δ14-reduction gives rise to a novel murine phenotype for which a corresponding human disorder has yet to be identified.
Niemann-Pick disease, type C1
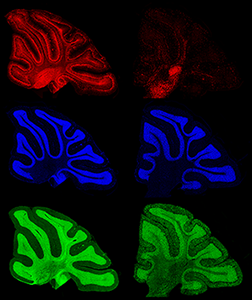
Figure 2. Comparison of control and late-stage NPC1 mouse cerebellum
Left: A sagittal cerebellar section from a control mouse is depicted, using fluorescent staining against a neuronal marker SMI32 (red), nuclear counterstain DAPI (blue), and lipid GM1 (green). Right: A comparable section from an Npc1–mutant mouse; note the extreme loss of Purkinje neurons (red), the perturbation of myelination (deep cerebellar tissue in green), and the green punctate accumulations of GM1 in the outermost cerebellar layer that are characteristic of the endolysosomal lipid storage found in this disorder.
Niemann-Pick disease, type C1 (NPC1) is a neurodegenerative disorder that results in ataxia and dementia. In view of the dementia, it has been referred to as childhood Alzheimer disease. The disorder is caused by a defect in intracellular lipid and cholesterol transport. Initially, as part of a Bench-to-Bedside award, we began a clinical protocol to identify and characterize biomarkers that could be used in a subsequent therapeutic trial. The project also received support from the Ara Parseghian Medical Research Foundation and Dana’s Angels Research Trust. We have enrolled over 75 NPC1 patients into a longitudinal Natural History trial. The goals of this trial are to identify (i) a blood-based diagnostic/screening test, (ii) biomarkers that can be used as tools to facilitate development and implementation of therapeutic trials, and (iii) clinical symptoms/signs that may be used as efficacy outcome measures in a therapeutic trial.
Currently, the average time from first symptom to diagnosis, the "diagnostic delay," in our cohort of NPC patients is on the order of four to five years. In collaboration with Daniel Ory, we found elevated levels of non-enzymatically produced oxysterols in NPC1 patients. As well as a potential biomarker that may be used to follow therapeutic interventions, the oxysterols will likely form the basis of a blood-based diagnostic test that will significantly shorten the diagnostic delay encountered by NPC1 patients.
In addition to our Natural History study, we completed a randomized, placebo-controlled, cross-over trial to investigate the safety and efficacy of N-acetyl cysteine (NAC) in NPC1. The goal was to determine whether NAC treatment would reduce oxidative stress and subsequently decrease levels of the non-enzymatically produced oxysterols. In collaboration with the Therapeutics of Rare and Neglected Disease Program of NCATS, we are conducting a phase I therapeutic trial of lumbar intrathecal cyclodextrin therapy in NPC1 and are working to initiate a multicenter, international clinical efficacy trial.
In collaboration with Daniel Ory and Frederick Maxfield, our group was awarded an NIH U01 grant to test the safety and potential efficacy of an HDAC inhibitor, vorinostat, in adult NPC1 patients. This collaboration also includes scientists from Notre Dame and has been supported by the Ara Parseghian Medical Research Foundation.
To complement the clinical work, we have begun to apply molecular and proteomic approaches to both mouse and human biomaterials to identify biological pathways disrupted in NPC1. We identified several blood and cerebral spinal fluid proteins and are in the process of validating the biomarkers as potential outcome measures to be used as tools in the development of therapeutic interventions.
Additional Funding
- U01HD079065 A Phase 1 Dose Escalation Study of Vorinostat in Niemann-Pick C1 Disease
- NICHD Directors Award: Phenotypic, electrophysiological investigation and treatment of hippocampal pathology in a mouse model of Niemann-Pick disease, type C1
- NICHD Directors Award: Identification and electrophysiological characterization of iPS derived neurons from Smith–Lemli–Opitz syndrome patients
Publications
- Porter FD, Scherrer DE, Lanier MH, Langmade SJ, Molugu V, Gale SE, Olzeski D, Sidhu R, Dietzen DJ, Fu R, Wassif CA, Yanjanin NM, Marso SP, House J, Vite C, Schaffer JE, Ory DS. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci Transl Med 2010;2:56ra81.
- Cluzeau CVM, Watkins-Chow DE, Fu R, Borate B, Yanjanin NM, Dail MK, Davidson CD, Walkley SU, Ory DS, Wassif CA, Pavan WJ, Porter D. Microarray expression analysis and identification of serum biomarkers for Niemann-Pick Disease, type C1. Hum Mol Genet 2012;21:3632-3646.
- Cologna SM, Jiang X-S, Backlund PS, Cluzeau CVM, Dail MK, Yanjanin NM, Siebel S, Toth CL, Jun H, Wassif CA, Yergey AL, Porter FD. Quantitative proteomic analysis of Niemann-Pick Disease, type C1 cerebellum identifies protein biomarkers and provides pathological insight. PloS One 2012;7(10):e478.
- Sparks SE, Wassif CA, Goodwin H, Conley SK, Lanham DC, Kratz LE, Hyland K, Gropman A, Tierney E, Porter FD. Decreased cerebral spinal fluid neurotransmitter levels in Smith-Lemli-Opitz syndrome. J Inherit Metab Dis 2014;37:415-420.
- Cross JL, Iben J, Simpson C, Thurm A, Swedo S, Tierney E, Bailey-Wilson J, Biesecker LG, Porter FD, Wassif CA. Determination of the allelic frequency in Smith-Lemli-Opitz syndrome by analysis of massively parallel sequencing data sets. Clin Genet 2014;E-pub ahead of print.
Collaborators
- Joan Bailey-Wilson, PhD, Inherited Diseases Research Branch, NHGRI, Bethesda, MD
- Paul Helquist, University of Notre Dame, Notre Dame, IN
- Frederick R. Maxfield, PhD, Weill Cornell Medical College, New York, NY
- Daniel Ory, MD, Washington University, St. Louis, MO
- Marc Patterson, MD, Mayo Clinic, Rochester, MN
- William Pavan, PhD, Genetic Disease Research Branch, NHGRI, Bethesda, MD
- Fran Platt, PhD, Oxford University, Oxford, United Kingdom
- Alan Remaley, MD, PhD, Molecular Diseases Branch, NHLBI, Bethesda, MD
- The Therapeutics for Rare & Neglected Diseases (TRND) Team, NCATS, NIH, Bethesda, MD
- Elaine Tierney, MD, Kennedy-Krieger Institute, Baltimore, MD
- Charles Vite, DVM, University of Pennsylvania, Philadelphia, PA
- Olaf G. Wiest, Dr rer nat, University of Notre Dame, Notre Dame, IN
- Alfred Yergey, PhD, Mass Spectrometry Core Facility, NICHD, Bethesda, MD
Contact
For more information, email fdporter@helix.nih.gov.