You are here: Home > Section on Epigenetics and Development
The X-chromosome and Gender Effects in Physiology, Pathophysiology, and Longevity

- Carolyn Bondy, MD, Head, Section on Epigenetics and Development
- Vladimir Bakalov, MD, Staff Clinician
- Clara Cheng, PhD, Staff Scientist
- Jian Zhou, MD, PhD, Staff Scientist
Some differences between normal women and men result from differential exposure to sex steroids. However, the effect does not adequately explain important differences in gender-specific susceptibility to disease and longevity. We propose that differential X-chromosome gene dosage contributes to fundamental biological differences between females and males. While researchers had considered the second X-chromosome in female cells entirely inert as a result of random X inactivation, the distinct phenotype of 45,X females with Turner syndrome (TS) indicates that the second X-chromosome is important for normal female development. Epigenetic mechanisms are involved in regional X inactivation as well as in genomic imprinting and thus contribute to sex differences by X-chromosome gene-dosage effects. Our research aims to identify and define the function of X-chromosome genes involved in the differential development and function of the brain and reproductive, metabolic, and immune systems in women and men. Studying girls and women with TS provides a unique opportunity to elucidate X-chromosome gene-dosage effects and deepen our understanding of TS, which affects approximately 1 in 2,000 females. Our research has improved our ability to care for girls and women with TS and enhance our understanding of women’s increased susceptibility to autoimmune disease and men’s elevated risk for coronary disease. This is the last year of this project as Dr. Bondy transitions to Scientist Emeritus in 2013.
X-chromosome, genomic imprinting, and longevity
Click image to enlarge.
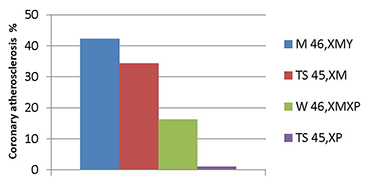
Figure 1. Coronary disease in Turner women monosomic for maternal vs. paternal X chromosomes compared with the male and female general population
Quantification of coronary atherosclerosis by CT angiography in middle-aged men (blue), Turner women with maternal X (red), normal women (green), and Turner women monosomic for a paternally derived (violet) X. The experiments are explained in the first section of the report.
Genomic imprinting involves the selective expression of certain genes, determined by parental origin. Genomic imprinting of X-linked genes causes biased expression in females and males, given that normal women are mosaic for maternally and paternally inherited X-chromosomes (XM and XP) while men are monosomic for XM. Women enjoy greater longevity than men mainly because of their lower risk, in younger age groups, for ischemic heart disease. Women’s primary advantage in this regard is their salutary “gynoid” fat distribution, that is, adipose tissue preferentially concentrated subcutaneously in the hips and thighs. In contrast, normal men tend to concentrate fat intra-abdominally, which has many adverse metabolic effects, including an atherogenic lipid profile and elevated mediators of inflammation, which cause coronary and cerebral artery atherosclerosis. To test the hypothesis that X-chromosome genomic imprinting contributes to the regional fat and metabolic differences between normal women and men, we studied the risk factors in groups of women monosomic for XM versus XP. The XM and XP groups had a similar BMI and total body fat, but women with XM had greater abdominal and intra-abdominal visceral fat, which were associated with higher LDL-cholesterol and triglycerides than in the XP group. Our finding of a male-type fat distribution and lipid profile in XM women supports the view that differential X-chromosome gene dosage, determined by genomic imprinting, contributes to the excess mortality from ischemic heart disease in 46,XMY men.
Very recently, we discovered that Turner women with a single maternally derived XM chromosome are at much greater risk for coronary artery atherosclerosis, as determined by CT coronary calcium scores and angiography, than women with a single paternally derived XP (Figure 1). Women monosomic for XM have a similar level of coronary atherosclerosis to the U.S. male population, as would be expected if parental imprinting of X chromosome genes impacted body composition, metabolism, and coronary risk. Women monosomic for XP, in contrast, appeared to be protected from coronary disease (see Figure 1), with a lower prevalence of coronary disease than ‘normal’ women who are mosaic for XMXP. This could be attributed to selective expression of a protective gene from XP, and we have now secured evidence for genomic imprinting of the X-linked gene OGT.
Congenital cardiovascular defects in Turner syndrome
Congenital cardiovascular disease may be the most serious medical problem in monosomy X or TS. Pioneering the use of high-resolution magnetic resonance angiography (MRA) for TS, we demonstrated cardiovascular anomalies in 50% of study subjects, in contrast to the previously accepted 25% prevalence based on echocardiographic studies. The most common anomaly disclosed by our study was a distinctive aortic deformation, termed elongated transverse arch of the aorta (ETA), affecting about 50% of women with TS. The newly described aortic abnormality appears to be predictive of aortic complications and hence mandates vigilant surveillance of aorta dimensions in affected yet asymptomatic patients. We reported the first prospective measure of the incidence of aortic dissection in TS and proposed new guidelines to identify high-risk patients (Matura et al., Circulation 2007;116:1663). During the study, we recorded three cases of aortic dissection among 158 patients, translating into an incidence of over 600 cases per 100,000 TS years compared with about 1 per 100,000 non–TS women years. The women who dissected were in their 40s and had aortic diameters from 3.7–4.8 cm. They were under cardiologist car, but, because their aortic diameter was less than 5 cm, they were not considered candidates for prophylactic intervention. We investigated various methods of normalizing MRI–measured aortic diameters to individual body size and found that aortic diameter/body surface area (Aortic Size Index, ASI) provided the greatest accuracy in identifying those at risk for dissection, with 33% of women with ASI greater than 2.5 cm/m 2 experiencing aortic dissection within approximately three years of follow-up. Thus, we established a new standard for evaluating aortic dilation and risk for dissection, normalized to the body size of at-risk patients, which identifies about 30% of women with TS who require close monitoring. If the ASI is equal to or greater than 2.5 cm/m2, the patient should be evaluated for prophylactic intervention. Further study is needed to determine whether a beta blocker or renin-angiotensin system blockade would prevent or retard aortic dilatation in TS patients and whether prophylactic surgery would reduce the incidence of aortic dissection and rupture. More recently, we characterized aortic valve morphology and function in relation to aortic diameters in about 250 girls and women with TS, showing that 3% have unicuspid, 23% bicuspid, 12% partially fused, and 64% normal tricuspid aortic valves. We also proved that the aortic valve defects were linked to deletion of the X chromosome short arm distal to Xp11.4 (4).
More recently, we examined the incidence of autoimmune diseases in 250 study subjects; we found that autoimmune thyroiditis was present in almost 40% of our TS population, compared with only about 5% of the general female population. Quite unexpectedly, during this focused analysis, we found that all women who had experienced serious aortic complications resulting in death or surgery had both autoimmune thyroiditis and bicuspid aortic valves (BAV). Further systematic investigation of this remarkable finding verified that the diagnosis of autoimmune thyroiditis is associated with progressive aortic dilation and that the combination of BAV and thyroiditis results in the greatest rate of aortic dilation and signifies a 66-fold greater risk of complications than for groups with either a single risk factor or neither. This important observation will greatly improve the ability to identify and appropriately guide and treat those at greatest risk, while reassuring those at little apparent risk. The data may also prove valuable for determining risk category for non-syndromic patients with the common congenital anomaly of BAV.
The X-chromosome, ovarian failure, and psychosocial function
Previous small surveys and anecdotal reports indicated that many girls and women with TS suffer from shyness and social anxiety. The “shyness trait” was traditionally attributed to stigmatization because of short stature (Cardoso et al., Gynecol Endocrinol 2004;19:313). More recently, researchers suggested that social difficulties may be neurobiologically based, caused by genomic imprinting of X-linked genes involved in “social cognition.” We showed that women with TS experience a rate of life-time depression similar to women from gynecologic clinic samples, with no “autistic spectrum” diagnoses. In open-ended interviews, girls and women identified ovarian failure, infertility, or “gender identity” as major issues related to their experience of TS. Thus, our findings suggest that the experience of ovarian failure is a major hurdle for older girls and women with TS, while major psychological diagnoses and autistic-type disorders are uncommon.
To investigate the behavioral impact of early ovarian failure, we compared psychosocial functioning in women with TS and women with 46,XX premature ovarian failure. We reasoned that, if the experience of premature ovarian failure per se leads to specific difficulties with social interactions in young women, the two groups should demonstrate similar responses on tests of psychosocial function. Women with normal ovarian function served as contemporaneous controls. Our two ovarian failure groups reported similar increases in shyness and social anxiety and lower self-esteem than did women with normal ovarian function. The findings suggest that the shyness and poor self-esteem observed in women with TS reflect the experience of premature ovarian failure. The feelings of social inadequacy are associated with social isolation, difficulty in partnering, and very limited sexual functioning. Current studies target the role of infertility, childlessness, sex steroid effects, or altered body image in impaired social functioning in women with TS. We will also address the issue of how best to inform a young woman and her family about the diagnosis of premature ovarian failure so as to minimize the psychological trauma. In addition, we plan to extend our studies to girls and young women who have survived cancer treatments that compromise ovarian function.
Our recent study on educational and professional outcomes in TS was spear-headed by a young woman with TS who participated as a study subject. Together with another woman with TS, a nurse, who was also a study participant, these researchers with a unique perspective on the syndrome posed the question as to how the parental origin of the single X chromosome impacts educational and occupational outcomes for women with TS. Together they analyzed 250 surveys completed by study subjects, which showed that 70% of our TS cohort had a baccalaureate or higher degree, compared with 26% of US women. The TS group was employed outside the home at a rate of 80%, compared with 70% in the U.S. female population. The TS population were significantly more drawn to healthcare and legal and social services occupations and significantly less likely to enter management, sales, and administrative support professions than the general female population. A paper describing the research is currently in press at the Journal of Women’s Health.
Additional Funding
- Bench to Bedside Award 2010-2012
Publications
- Grafodatskaya D, Chung BH, Butcher DT, Turinsky AL, Goodman SJ, Choufani S, Chen YA, Lou Y, Zhao C, Rajendram R, Abidi FE, Skinner C, Stavropoulos J, Bondy CA, Hamilton J, Wodak S, Scherer SW, Schwartz CE, Weksberg R. Multilocus loss of DNA methylation in individuals with mutations in the histone H3 lysine 4 demethylase KDM5C. BMC Med Genomics 2013;6:1.
- Gould HN, Bakalov VK, Tankersley C, Bondy CA. High levels of education and employment among women with turner syndrome. J Womens Health (Larchmt) 2013;22(3):230-235.
- Olivieri LJ, Baba RY, Arai AE, Bandettini WP, Rosing DR, Bakalov V, Sachdev V, Bondy CA. Spectrum of aortic valve abnormalities associated with aortic dilation across age groups in Turner syndrome. Circ Cardiovasc Imaging 2013;6(6):1018-1023.
- Bondy C, Bakalov VK, Cheng C, Olivieri L, Rosing DR, Arai AE. Bicuspid aortic valve and aortic coarctation are linked to deletion of the X chromosome short arm in Turner syndrome. J Med Genet 2013;50(10):662-665.
- Prakash S, Guo D, Maslen CL, Silberbach M, Milewicz D, Bondy CA. Single-nucleotide polymorphism array genotyping is equivalent to metaphase cytogenetics for diagnosis of Turner syndrome. Genet Med 2013;Epub ahead of print.
Collaborators
- Andrew Arai, MD, Laboratory of Cardiac Energetics, NHLBI, Bethesda, MD
- Ahmed Gharib, MB, ChB, Biomedical and Metabolic Imaging Branch, NIDDK, Bethesda, MD
- Dianna Milewicz, MD, PhD, The University of Texas Health Science Center at Houston, Houston, TX
- Douglas Rosing, MD, Cardiology Branch, NHLBI, Bethesda, MD
- Vandana Sachdev, MD, Cardiovascular Branch, NHLBI, Bethesda, MD
- Rosanna Weksberg, MD, PhD, The Hospital for Sick Children, Toronto, Canada
Contact
For further information, contact bondyc@mail.nih.gov.

