You are here: Home > Section on Neuroendocrinology
Pineal Gland, Chronobiology, and Neuroepigenetics

- David C. Klein, PhD, Head, Section on Neuroendocrinology
- Sam Clokie, PhD, Research Fellow
- Steven L. Coon, PhD, Staff Scientist
- Hyun-Hee Kim, PhD, Visiting Fellow
- Masahiro Matsuo, MD, Visiting Fellow
- Cong Fu, PhD, Visiting Fellow
- M.A.A. Namboodiri, PhD, Guest Researcher
- Harvey Pollard, MD, PhD, Guest Researcher
- Fumiyoshi Yamazaki, PhD, Visiting Fellow
- Susan Yudiskaya, MD, Special Volunteer
We study the pineal gland and regulation of the production of the pineal hormone melatonin. The work has broad implications for vertebrate biology and is of special interest to clinical scientists studying human diseases relating to circadian rhythms, including endocrine pathologies, sleep and mood disorders, and deficiencies in alertness. The Section also addresses the factors that control the remarkable global changes in gene expression occurring in the pineal gland on a 24-hour basis as well as factors associated with the establishment of the pineal phenotype. Interests range from small and large non-coding RNAs to mRNAs. The Section is dedicated to advancing understanding of pineal function through the use of high-throughput sequencing to examine the pineal transcriptome in model experimental animals, including zebra fish, chicken, mouse, rat, and rhesus monkey, and to compare these profiles with those of the human pineal transcriptome. The goal of this effort is to identify core and highly conserved elements of pineal biology that can be optimally studied using resources available to the program. Moreover, we are involved in efforts to apply such methods to other systems to better understand the molecular basis of biological regulation.
The timezyme: arylalkylamine N-acetyltransferase
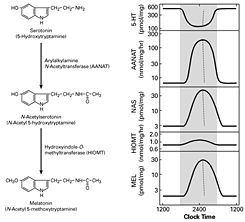
Figure 1. Daily rhythm in indole metabolism in the pineal gland
The daily rhythm in circulating melatonin production reflects the increased production in the pineal gland, as depicted here. During the day, melatonin production is low owing to low levels of AANAT. At night, AANAT activity rises, resulting in an increase in N-acetylserotonin. The increase in N-acetylserotonin raises melatonin production by a mass action effect; the level of the last enzyme in melatonin synthesis is constant. Changes in melatonin synthesis cause parallel changes in melatonin release. Circulating melatonin is rapidly destroyed in the liver, which allows changes in production and release to be immediately reflected in changes in circulating melatonin.
A pivotal advance in our investigations into arylalkylamineN-acetyltransferase (AANAT), the second and penultimate enzyme in the pathway for melatonin synthesis from serotonin, was our finding that the enzyme is critical to the control of the rhythm in melatonin synthesis (Figure 1). In all species examined to date, the large increase in melatonin synthesis at night is a highly conserved feature of vertebrate physiology.
The transcriptional mechanisms that control expression of the Aanat gene in rodents and the chicken include interactions of cyclic AMP–response elements in the promoter region (1); other response elements in the promoter, including the E-box and photoreceptor conserved elements, appear to determine the marked tissue-specific pattern of expression of the gene, which is limited to the pineal gland and retina. We determined that, in rodents, expression of Aanat rises about 100-fold at night; however, this feature of regulation is not highly conserved. We discovered little or no nocturnal increase in Aanat expression in ungulates and primates. Thus, transcriptional control mechanisms are not essential for regulating melatonin synthesis in all vertebrates.
With regard to highly conserved post-translational control of AANAT, we and our collaborators determined that it is mediated by cyclic AMP and involves phosphorylation at C- and N-terminal sites by cyclic AMP–dependent protein kinase. This leads to formation of a reversible complex with 14-3-3 protein, in which the enzyme is stabilized and activated, as indicated by structural, in vivo, and in vitro studies. When cyclic AMP levels fall, AANAT is dephosphorylated and destroyed by the proteasome.
Together with our collaborators, we recently determined that the form of AANAT found in the pineal gland evolved very rapidly approximately 500 million years ago from an enzyme dedicated to detoxification; this ancestor lacked the regulatory and catalytic features that are characteristic of the regulation and production of melatonin in the pineal gland. The evolutionary change—termed neofunctionalization—coincided with the appearance of the pineal gland and lateral eyes. The findings support the hypothesis that the lateral eyes and the pineal gland evolved from a common primitive photodetector and that this was facilitated by the primitive form of AANAT, which served to enhance photodetection by removing toxic arylalkylamines. The elevated activity at night is likely to have enhanced the ability to detect low levels of light. Subsequently, one of the downstream products of the acetylation, melatonin, was recognized as a unique and valuable signal of night time, a time-keeping signal, which promoted the independent evolution of the eyes dedicated to image detection and the pineal gland dedicated to melatonin production and time keeping.
Neuroepigenetics: global control of daily changes in pineal gene expression are regulated by an adrenergic/cyclic AMP mechanism.
Transcriptome profiling:
We initiated several projects aimed at obtaining a global picture of differences in gene expression that occur on a night/day basis and identifying genes that are highly enriched in the pineal gland. In collaboration with Igor Dawid, Yoav Gothilf, and Reiko Toyama, we characterized pineal gene expression in the zebrafish and other species as a function of time of day and of development. We had already identified a set of genes highly expressed in the pineal gland. In collaboration with Peter Munson, David Carter, and Ruben Baler, we analyzed the control of gene expression in second-messenger cascades (see J Biol Chem 2009;284:7606). The work triggered several investigations that analyze the expression of genes not previously reported in the pineal literature, including those encoding methionine adenosyl transferase, MAP kinase phosphatase-1, PEPT1, phoshodiesterase 4D2, the dopamine Drd4 receptor, a subunit of the IgE receptor, and Rgs4.
The group of pineal-specific genes expressed in the pineal gland of several vertebrates includes genes known to be associated with melatonin production and visual signal transduction. In addition, we identified many genes (over 600), new to the pineal literature, that exhibit more than two-fold night/day differences in expression and another set of genes that are more highly expressed in the pineal gland than in other tissues. The work is leading to a rapid increase in knowledge of the biochemical profile, conserved across species, of the pineal gland. It is also pointing to new transcriptional pathways controlled by previously unrecognized transcription factors; by analyzing the transcription factors and the promoters of genes either upregulated at night or highly expressed in the pineal gland, we will be able to construct a regulatory network that describes the cascade of transcription factors underlying the control of pineal gene expression. We also identified new and potentially important pathways involved in cell-to-cell communication and signal transduction in the pineal gland; expression of some genes points to new functions for the pineal gland.
Using quantitative RT-PCR and radiochemical in situ hybridization, our collaborators David Sugden, Morten Møller, and Martin Rath confirmed the results of microarray studies on the rat pineal gland, providing a more detailed and quantitative description of the 24-hour patterns of gene expression and revealing that the patterns are not all similar or in phase but rather exhibit novel features. Our results permit a full-scale biochemical and physiological analysis of the control of genes in the pineal gland. Results from organ culture work revealed that the vast majority of genes that exhibit night/day changes in expression are also regulated by norepinephrine, acting through a cyclic AMP mechanism that may involve cyclic AMP–response element binding sites in regulated genes and less specific epigenetic mechanisms, affecting many processes (Figure 2).
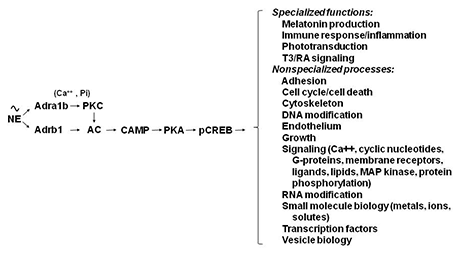
Figure 2. Neural regulation of gene expression in the rat pineal gland
Sympathetic nerves release norepinephrine (NE), which binds to beta1-adrenergic receptors (Adrb1) and to alpha1b-adrenergic receptors (Adra1b). NE acts through the Adrb1 receptor to partially activate adenylate cyclase (AC) and through Adra1b to potentiate activation of the Adrb1 receptor. The interaction involves Adra1b–dependent activation of Ca2+ and phosphatidylinositol (Pi) activation of protein kinase C (PKC), which enhances Adrb1 stimulation of AC. The resulting increase in cyclic AMP (CAMP) leads to activation of protein kinase A (PKA), which phosphorylates cyclic AMP–response element binding protein (CREB). The consequence of this is the global shift in gene expression, as illustrated in Figure 1. The transcription factor profile unique to this tissue establishes which genes are regulated by CREB phosphorylation.
RNA sequencing:
Transcriptome profiling has been extended using high-throughput/next-generation sequencing, which confirmed many of the indications from microarray and revealed previously unrealized expressed introns and flanking regions. We characterized daily changes in transcripts in the pineal gland and retina and changes that occur during the course of development. In addition, the effort led to the characterization of developmental dynamics in hundreds of transcription factors expressed in the pineal gland. This advance will serve as a foundation for future studies on the mechanisms that control developmental changes in the pineal gland and determine fate and those that function in the adult to control 24 hour changes in expression of genes.
Long noncoding RNAs:
RNA sequencing identified a set of highly tissue-specific and rhythmically expressed non-coding RNAs. The transcripts range in size from approximately 200 bp to over 10,000 bp and vary in their genomic location from genetic deserts to being transcribed from introns of known genes. In at least one case, a long noncoding RNA overlaps a known gene and, in another, the long noncoding RNA is transcribed from the strand of DNA that is opposite to that encoding a known gene. The expression of these interesting molecules is regulated by the same neural system that controls coding transcripts. The transcripts may function in biological regulation through interaction with DNA or proteins.
MicroRNAs (miRNAs):
miRNAs play a broad range of roles in biological regulation. We profiled rat pineal miRNAs for the first time and evaluated their importance by focusing on the main function of the pineal gland, melatonin synthesis. Massively parallel sequencing and related methods revealed that the miRNA population is dominated by a small group of miRNAs as follows: about 75% is accounted for by 15 miRNAs; miR-182 represents 28%. In addition to miR-182, miR-183 and miR-96 are also highly enriched in the pineal gland, a distinctive pattern also found in the retina. The effort also identified previously unrecognized miRNAs and other small noncoding RNAs. Pineal miRNAs do not exhibit a marked night/day differences in abundance with few exceptions (e.g., two-fold night/day differences in the abundance of miR-96 and miR-182), in sharp contrast with the dynamic 24-h pattern that characterizes the pineal transcriptome. During development, the abundance of most pineal gland–enriched miRNAs increases; however, there is a marked decline in at least one, miR-483. Based on the following observation, miR-483 is a likely regulator of melatonin synthesis: it inhibits melatonin synthesis by pinealocytes in culture; it acts via predicted binding sites in the 3′ UTR of Aanat mRNA; and it exhibits the reverse developmental profile to that of Aanat transcripts. Additionally, a miR-483–targeted antagonist increased melatonin synthesis in neonatal pinealocytes. The observations support the hypothesis that miR-483 suppresses Aanat mRNA levels during development and that the developmental decline in miR-483 abundance promotes melatonin synthesis.
New element in biological timing:
We used the zebrafish pineal gland to study several aspects of melatonin production, including the nature of the biological clock. As is true of all sub-mammalian vertebrates, the zebrafish pineal gland contains a strong biological clock linked to melatonin production and a photodection system that mediates effects of light on the clock and on melatonin production. Our analysis of the zebrafish pineal gland, which employed both microarray and RNA sequencing, identified a new element in chronobiology, camk1gb, which is driven by the biological clock and links the clock with locomotor activity.
Negative feedback control mechanisms:
Melatonin production is regulated by norepinephrine acting through G protein–linked receptors. We found that the abundance of regulator of G-protein signaling 2 (RGS2) increases at night, that expression is augmented by norepinephrine, and that the protein has a negative feedback effect on melatonin production. The data are consistent with the conclusion that RGS2 functions on a daily basis to negatively modulate melatonin production.
Impact of RNA sequencing
The Section has been successful in promoting the use of RNA sequencing in programs not involved in pineal research, including work with Michael Iadarola on pain pathways, Greti Aguilera on the hypothalamus, and Stanko Stojilkovic on the pituitary gland. In the latter case, RNA sequencing and our subsequent analysis revealed that the hypothalamic peptide GnRH induces a greater than 600-fold increase in expression of dentine matrix protein-1. The finding provided the foundation for a large body of work by Stojilkovic's lab, which established that the response took place in the gonadotroph, was mediated by the GnRH receptor, and occurred following puberty. It was also found that the response is less than 5% as robust in males as in females, and that expression of the gene was observed in vivo only during ovulation. Dentine matrix protein-1 had not previously appeared in the neuroendocrine literature, underscoring the potential impact that application of RNA sequencing can have on our understanding of biological mechanisms.
Additional Funding
- United States–Israel Binational Science Foundation grant numbers 2009290 and 2005280
Publications
- Falcón J, Coon SL, Besseau L, Cazaméa-Catalan D, Fuentès M, Magnanou E, Paulin C-H, Boeuf G, Sauzet S, Jorgensen EH, Mazan S, Wolf Y, Koonin EV, Steinbach PJ, Hyodo S, Klein, DC. Drastic neofunctionalization associated with evolution of the "timezyme" AANAT 500 million years ago. Proc Natl Acad Sci USA 2013;in press.
- Yamazaki F, Kim H-H, Lau P, Hwqant CK, Iuvone PM, Klein DC, Clokie SJH. pY RNA1-s2: a highly retina-enriched small RNA that selectively binds to Matrin 3 (Matr3). PLoS One 2013;in press.
- Matsuo M, Coon SL, Klein DC. RGS2 is a feedback inhibitor of melatonin production in the pineal gland. FEBS Lett 2013;587:1392-1398.
- Kucka M, Bjelobaba I, Clokie SJ, Klein DC, Stojilkovic SS. Female-specific induction of rat pituitary dentin matrix protein-1 by GnRH. Mol Endocrinol 2013;27:1840-1850.
- Rath MF, Rohde K, Klein DC, Møller M. Homeobox genes in the rodent pineal gland: roles in development and phenotype maintenance. Neurochem Res. 2013;38:1100-1112.
Collaborators
- Greti Aguilera, PhD, Program on Developmental Endocrinology and Genetics, NICHD, Bethesda, MD
- Ruben Baler, PhD, Science Policy Branch, NIDA, Rockville, MD
- David Carter, PhD, University of Wales, Cardiff, UK
- Praveen Cherukuri, PhD, Genome Technology Branch, NHGRI, Rockville, MD
- Igor Dawid, PhD, Program in Genomics of Differentiation, NICHD, Bethesda, MD
- Jack Falcón, PhD, Observeratoire Oceanologique/CNRS, Banyuls-sur-Mer, France
- Sandra Goebbels, PhD, Max-Planck-Institut für Experimentelle Medizin, Göttingen, Germany
- Yoav Gothilf, PhD, Tel Aviv University, Tel Aviv, Israel
- Stephen Hartley, PhD, NHGRI, Rockville, MD
- Michael Iadarola, PhD, Laboratory of Sensory Biology, NIDCR, Bethesda, MD
- P. Michael Iuvone, PhD, Emory University School of Medicine, Atlanta, GA
- Eugene Koonin, PhD, National Center for Biotechnology Information, NLM, Bethesda, MD
- Morten Møller, MD PhD, Panum Institute, University of Copenhagen, Copenhagen, Denmark
- Jim Mullikin, PhD, Genome Technology Branch, NHGRI, Rockville, MD
- Estela Muñoz, PhD, Instituto de Histología y Embriología, Universidad Nacional de Cuyo, Mendoza, Argentina
- Peter Munson, PhD, Mathematical and Statistical Computing Laboratory, CIT, NIH, Bethesda, MD
- Martin F. Rath, PhD, Panum Institute, Copenhagen University, Copenhagen, Denmark
- Leming Shi, PhD, Fudan University, Shanghai, China
- Stanko Stojilkovic, PhD, Program in Developmental Neuroscience, NICHD, Bethesda, MD
- David Sugden, PhD, Kings College, University of London, London, UK
- Reiko Toyama, PhD, Program in Genomics of Differentiation, NICHD, Bethesda, MD
- Thomas Werner, PhD, University of Michigan, Ann Arbor, MI, and Genomatix, Munich, Germany
- Yuri Wolf, PhD, National Center for Biotechnology Information, NLM, Bethesda, MD
Contact
For more information, email kleind@mail.nih.gov or visit sne.nichd.nih.gov.