You are here: Home > Section on Growth and Obesity
Physiology, Psychology, and Genetics of Obesity

- Jack A. Yanovski, MD, PhD, Head, Section on Growth and Obesity
- Joan C. Han, MD, Assistant Clinical Investigator
- Bonggi Lee, PhD, Visiting Fellow
- Roya Sherafat, MD, PhD, Visiting Fellow
- Tania Condarco, MD, Fellow, Adult Endocrine Training Program
- Andrew Demidowich, MD, Fellow, Adult Endocrine Training Program
- Sarah Berger, PhD, Special Volunteer
- Lauren B. Shomaker, PhD, Special Volunteer
- Sheila M. Brady, RN, FNP, Nurse Practitioner
- Ryan Gardner, BA, Postbaccalaureate Fellow
- Amanda Krause, BS, Postbaccalaureate Fellow
- Mira Mooreville, BA, Postbaccalaureate Fellow
- Samantha Reina, BA, Postbaccalaureate Fellow
- Nicole Sedaka, BS, Postbaccalaureate Fellow
- Dezmond Taylor-Douglas, BS, Graduate Student
The prevalence of overweight and obesity in children and adults has tripled during the past 40 years (1). The alarming rise in body weight has likely occurred because the current environment affords easy access to calorie-dense foods and requires less voluntary energy expenditure. However, this environment leads to obesity only in those individuals whose body weight–regulatory systems are not able to control body adiposity with sufficient precision in our high calorie/low activity environment, which suggests there are subgroups in the US with a uniquely high susceptibility to weight gain under the prevailing environmental conditions. Our primary goal is to elucidate the genetic underpinnings of the metabolic and behavioral endo-phenotypes that contribute to the development of obesity in children. Using our unique longitudinal cohort of children at risk for adult obesity, who have undergone intensive metabolic and behavioral phenotyping, we examine genetic and phenotypic factors predictive of progression to adult obesity in children who are in the “pre-obese” state, allowing characterization of phenotypes unconfounded by the impact of obesity itself. Once identified as linked to obesity, we study intensively genetic variants that impair gene function. We expect that these approaches will improve our ability to predict which children are at greatest risk for obesity and its comorbid conditions and will lead to more targeted, etiology-based prevention and treatment strategies for pediatric obesity.
Genetic factors important for childhood body weight regulation
Click image to enlarge.
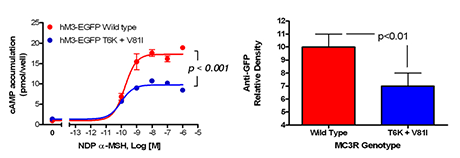
Figure 1
Studies of a human MC3R variant containing two naturally occurring polymorphisms—a variant associated with pediatric-onset obesity—found that the variant was partially inactive, with diminished signal transduction (left panel), likely the result of reduced protein expression (right panel).
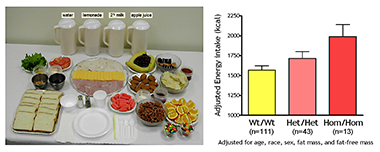
To identify gene variants affecting body composition, we have been examining polymorphisms in genes involved in the leptin signaling pathway. Genes include the leptin receptor FTO and those encoding proopiomelanocortin (POMC), the melanocortin 3 receptor (MC3R), brain-derived neurotrophic factor (BDNF), and histaminergic receptors 1 and 3. We are currently studying a variant MC3R that is associated with adiposity in children and appears to have functional significance for MC3R signal transduction. Children who were homozygous variant for both Thr6Lys and Val81Ile polymorphisms have significantly greater BMI-SD scores, fat mass, and body circumference measurements and higher plasma levels of insulin and leptin than unaffected or heterozygous children. In vitro studies subsequently found that signal transduction and protein expression were significantly lower for the double mutant MC3R (Figure 1). Our ongoing studies attempt to understand the mechanisms by which these sequence alterations may affect body weight. We found that children homozygous variant for both Thr6Lys and Val81Ile polymorphisms showed no deficits in energy expenditure but demonstrated hyperphagia in laboratory meal studies (Figure 2). The results were specific to function-altering mutations and not associated with other common polymorphisms that we identified in the MC3R. Transgenic “knock-in” mice expressing the human wild-type and human double-mutant MC3R were therefore developed and are currently under investigation. Preliminary analyses suggest alterations in body composition consistent with our observations in humans. These mice will be intensively phenotyped over the next two years. We have also initiated a clinical investigation of lipolysis and lipogenesis in humans with these polymorphisms.
Figure 2
Energy intake is studied by using free-access buffet meals of palatable foods. Children homozygous for two polymorphisms in the MC3R gene consumed more at the buffet than heterozygotes or those with wild-type MC3R.
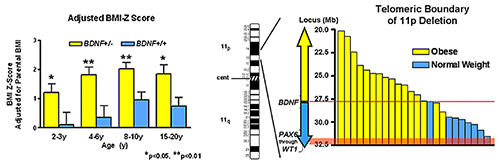
We also investigated the BDNF-TrkB (brain-derived neurotrophic factor/tropomyosin receptor kinase B) pathway in relation to body mass in children. In a cohort of 328 children, age 3–19 years, we found obese children exhibited significantly lower BDNF levels; BMI, BMI z score, and body fat were all negatively associated with BDNF. These data suggest that some obese individuals with low serum BDNF for age may have mutations that alter BDNF function. We therefore assessed the role of BDNF haploinsufficiency as a cause of obesity in patients with syndromes attributable to deletions in the vicinity of 11p14.1, where the human BDNF gene is located. In 33 subjects with the WAGR (Wilms' tumor, aniridia, genitourinary, and renal abnormalities) syndrome who had heterozygous 11p deletions, ranging in size from 1.0–26.5 Mb, 19 had regions of deletion that involved the BDNF gene (BDNF+/−). Compared with individuals with intact BDNF (BDNF+/+), BDNF+/− had significantly greater body mass during childhood, starting at age two (Figure 3).
Click image to enlarge.
Figure 3
Patients with WAGR syndrome who have haploinsufficiency for brain-derived neurotrophic factor (BDNF) had a higher BMI standard deviation score (BMI Z score) than children and adults with WAGR syndrome who retained two copies of the BDNF gene (left panel). Deletions that extended into exon 1 of BDNF were associated with 100% risk of childhood-onset obesity (right panel).
We have also been investigating conditions such as Bardet-Biedl and Alström syndromes that, based on mouse model data, may involve impaired leptin receptor function. In collaboration with Leslie Biesecker’s group, we recently reported that hyperphagia (2) was prevalent among patients with Bardet-Biedl syndrome, and we previously found evidence for hyperleptinemia among such patients that was consistent with leptin-receptor resistance, suggesting that defect-specific therapy may be possible.
Physiology, metabolism, and psychology of childhood body weight regulation
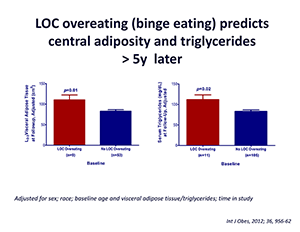
Our studies are directed at understanding the physiological, psychological, and metabolic factors that place children at risk for undue weight gain. In order to determine the factors that are most important for development of the complications of obesity in youth, we study normal-weight children and adolescents, children who are already obese, and the non-obese children of obese parents. Body composition, leptin concentration, metabolic rate, insulin sensitivity, glucose disposal, energy intake at buffet meals, and genetic factors believed to regulate metabolic rate and body composition are examined. Psychological and behavioral factors, such as propensity to engage in binge-eating behavior (Figure 4), are also studied (3). Children are being followed longitudinally into adulthood.
Figure 4
On average (±SE), children who engage in binge eating at baseline have greater visceral adipose tissue at L2-3 at follow-up than children who do not engage in binge eating at baseline, adjusting for sex, race, baseline age, baseline visceral adipose tissue at L2-3, and time in study (P = 0.01).
On average (±SE), children who engage in binge eating at baseline have higher follow-up triglycerides than children who do not engage in binge eating at baseline, adjusting for sex, race, baseline age, body mass index (kg/m2), baseline triglycerides, and time in study (P = 0.02).
As part of these studies, we examined how best to measure eating-related psychopathology, insulin sensitivity, changes in body composition, energy intake, and energy expenditure in children and we studied the short- and long-term stability of the components of metabolic syndrome. We found that leptin is an important predictor of weight gain in children: those with elevated leptin levels gain even more weight when followed longitudinally than do children with normal leptin levels. We also documented that hyperinsulinemia is positively related to energy intake in non-diabetic, obese children. We also examined the relationship between depressive symptomatology and insulin resistance in children and adolescents and found strong associations, both cross-sectionally and prospectively, between depressive symptoms and insulin resistance independent of body weight. The associations suggest mechanisms whereby insulin resistance may contribute to excessive weight gain in children and have informed some of our treatment approaches to pediatric obesity (described below).
Our evaluations concentrating on binge eating behaviors in children suggest that such behaviors also are associated with adiposity in children. We also found that binge-eating and dieting behaviors may predict future weight gain in children at risk for obesity: children reporting binge-eating behaviors, such as loss of control (LOC) over eating, gained, on average, an 2.4 kg more weight per year than did non-binge-eating children; actual intake during buffet meals averaged 400 kcal more in children engaging in binge eating, but despite their greater intake, such children reported shorter-lived satiety than children not engaging in binge-eating episodes. The ability to consume large quantities of palatable foods, especially when coupled with reduced subsequent satiety, may play a role in the greater weight gain found in binge-eating children. The data also suggest that interventions targeting disordered eating behaviors may be useful in preventing excessive fat gain in children prone to obesity and have led to ongoing trials to study preventative strategies related to binge eating.
Because binge eating appears to be a heritable trait, we also initiated studies to investigate potential genetic factors linked to LOC over eating. For example, we previously reported among 229 youth aged 6–19 y who were genotyped for FTO SNP rs9939609, subjects with at least one A allele, the risk allele, (67.7%) had significantly elevated BMI, BMI z scores and fat mass compared with subjects with TT (normal) alleles. Of the AA/AT subjects, 34.7% reported LOC compared with 18.2% of the TT subjects. AA/AT subjects consumed a greater percentage of energy from fat than did the TT subjects. We recently found preliminary evidence of a link between FTO SNP rs9939609 and eating in the absence of hunger, evidence that will be pursued further in the next year.
In two protocols, we study actual food consumption of children during meals, to elucidate differences in the calorie and macronutrient content of meals and the circulating hormones related to hunger and satiety in those who either endorse binge-eating behaviors or report no such behaviors. We found that eating in the absence of physiological hunger is a replicable trait that appears linked to obesity. We hypothesize that differences in these factors will predict the development of obesity in the populations studied and may be of great importance in developing rational approaches for the prevention and treatment of obesity in the diverse US population. A new clinical protocol based on a successful pilot study is examining the effects of a targeted interpersonal therapy intervention on body weight change in adolescents who endorse binge-eating behaviors.
Treatment of obesity and the co-morbid conditions associated with obesity
Given the rapid increase in the prevalence of obesity, the development of treatments for obesity in children and adults is urgently needed, yet current pharmacologic approaches are extremely limited. In several clinical protocols, we examined approaches for the prevention and treatment of excessive body weight. We completed a pilot study demonstrating that severely obese adolescents can lose weight when enrolled in a comprehensive weight-management program that includes the gastrointestinal lipase inhibitor orlistat as an adjunct to a behavior-modification program. We also completed a placebo-controlled randomized trial, studying whether the weight loss of African American and Caucasian children and adolescents who have obesity-related comorbidities was improved by the use of orlistat 120 mg three times a day. Subjects participated in a 12-week weight reduction program. We compared body weight and body composition [by dual-energy X-ray absorptiometry (DXA) and air displacement plethysmography], glucose homeostasis by frequently sampled intravenous glucose tolerance test (FSIGT), fasting lipids, pulse, and blood pressure before and after treatment. We studied 200 adolescents (130 female, 122 African American, mean age ± SEM 14.6 ± 0.10y, BMI 41.7 ± 0.6 kg/m2, range 27–87 kg/m2), with over 85% of subjects completing the trial. Adolescents treated with orlistat lost more weight, BMI units, and fat mass than untreated individuals. Although pulse and blood pressure fell during the trial, orlistat treatment did not significantly alter these variables. Similarly, HOMA-IR (homeostasic model of assessment-insulin resistance) , SI (insulin sensitivity index) by FSIGT, Apo B (apolipoprotein B), total and LDL-cholesterol, and triglycerides fell in proportion to weight loss, but orlistat use was not associated with significant reductions in any of these obesity-related laboratory comorbidities. Both AST and ALT unexpectedly increased significantly with orlistat treatment. We concluded that orlistat added to a behavioral program significantly improved weight loss over a 6-month interval, but had little effect on obesity-related co-morbid conditions in obese adolescents.
We also took advantage of this trial to study free fatty acid (FFA) flux among obese boys and girls (4). In adolescents, it was not known whether sex-associated differences in FFA flux occur. We therefore studied FFA kinetics in 112 non-Hispanic white and black adolescents (38 males, 74 females; age range 12–18 years; body mass index SD score range 1.6-3.1). Glucose, insulin, and FFA were measured during insulin-modified, frequently sampled iv glucose tolerance tests. Minimal analytical models for glucose and FFA calculated insulin sensitivity index (SI) and FFA kinetics. We examined relationships of FFA measures to sex, resting energy expenditure (REE), fat mass (FM), lean body mass (LBM), and visceral adipose tissue (VAT). In models accounting for age, race, pubertal status, height, FM, and LBM, we found that, independent of the effects of REE and FM, FFA kinetics differ significantly in obese adolescent girls and boys, suggesting greater FFA flux among girls.
A second obesity treatment study examined the mechanism by which metformin affects the body weight of younger children who have hyperinsulinemia and are therefore at risk for later development of type 2 diabetes. We conducted a single-center, 6-month, randomized, double-blind, placebo-controlled trial of the effects of metformin, 1000 mg twice a day, administered with meals, in severely obese children (6–12y) who manifested hyperinsulinemia and insulin resistance. Subjects participated in a monthly dietitian-administered weight reduction program. Body mass index and body composition (by air displacement plethysmography), glucose homeostasis (by HOMA-IR), and lipids were measured before and after six months of treatment. One hundred obese children (60 females, 11 Hispanic, 3 Asian, 40 African American), mean age 10.2±1.5y, with mean BMI 34.6±6.6 kg/m2 (range 23-58 kg/m2) were enrolled between October, 2000, and April, 2007. 85 of the subjects (84% given metformin and 86% given placebo) completed the trial. Of those randomized to metformin, their BMI, BMI z score, and body fat mass declined to a significantly greater extent than did those of placebo-treated children. Serum glucose and HOMA-IR also decreased more in metformin-treated than in placebo-treated children. We concluded that metformin, added to a monthly behavioral program, significantly improved weight loss and insulin resistance over a 6-month interval in severely obese, insulin-resistant children.
A third clinical trial (5) examined efficacy of two diets for Hispanic children and adolescents, among whom the prevalence of obesity and insulin resistance is considerably greater than among non-Hispanic white children. A low glycemic-load diet (LGD) has been proposed as an effective dietary intervention for pediatric obesity, but no published study had examined the effects of an LGD in obese Hispanic children. We compared the effects of an LGD and a low-fat diet (LFD) on body composition and components of metabolic syndrome in obese Hispanic youth. Obese Hispanic children (7–15 years of age) were randomly assigned to consume an LGD or an LFD in a two-year intervention program. We obtained body composition and laboratory assessments at baseline and 3, 12, and 24 months after intervention. In the 113 children who were randomly assigned, 79% of both groups completed three months of treatment and 58% of LGD and 55% of LFD subjects attended 24-month follow-up. Compared with the LFD, the LGD reduced the glycemic load per kilocalories of reported food intakes in participants at three months. Both groups had a lowered BMI z score, which was expressed as a standard z score relative to CDC age- and sex-specific norms, improved waist circumference, and systolic blood pressure at 3, 12, and 24 months after intervention. However, there were no significant differences between groups for changes in BMI, insulin resistance, or components of metabolic syndrome. We found no evidence that an LGD and an LFD differ in efficacy for the reduction of BMI or aspects of metabolic syndrome in obese Hispanic youth. Both diets reduced the BMI z score when prescribed in the context of a culturally adapted, comprehensive weight-reduction program.
A fourth recently completed study examined the role of central nervous system histamine in controlling food intake at meals. An additional ongoing study tests methods to reduce insulin resistance in adolescents at risk for Type 2 Diabetes. This protocol should complete accrual in 2014.
Additional Funding
- Prader-Willi Syndrome Association (USA) Best Idea Grant for Hyperphagia Research
- NIH Clinical Center "Bench to Bedside" Award: Fat Metabolism and Function-Altering Polymorphisms in MC3R 2011–2013
- NIH Clinical Center "Bench to Bedside" Award: Depression and Insulin Resistance in Adolescent Girls 2011–2013
- Office of Disease Prevention, NIH: Grant supplement to support clinical protocol “Mood and Insulin Resistance in Adolescents at Risk for Diabetes”
Publications
- Yanovski SZ, Yanovski JA. Obesity prevalence in the United States—up, down, or sideways? N Engl J Med 2011;364:987-989.
- Sherafat-Kazemzadeh R, Ivey L, Kahn SR, Sapp JC, Hicks MD, Kim RC, Krause AJ, Shomaker LB, Biesecker LG, Han JC, Yanovski JA. Hyperphagia among patients with Bardet-Biedl syndrome. Pediatr Obes 2013;8:e64-67.
- Tanofsky-Kraff M, Shomaker LB, Stern EA, Miller R, Sebring N, Dellavalle D, Yanovski SZ, Hubbard VS, Yanovski JA. Children's binge eating and development of metabolic syndrome. Int J Obes (Lond) 2012;36:956-962.
- Adler-Wailes DC, Periwal V, Ali AH, Brady SM, McDuffie JR, Uwaifo GI, Tanofsky-Kraff M, Salaita CG, Hubbard VS, Reynolds JC, Chow CC, Sumner AE, Yanovski JA. Sex-associated differences in free fatty acid flux of obese adolescents. J Clin Endocrinol Metab 2013;98:1676-1684.
- Mirza NM, Palmer MG, Sinclair KB, McCarter R, He J, Ebbeling CB, Ludwig DS, Yanovski JA. Effects of a low glycemic load or a low-fat dietary intervention on body weight in obese Hispanic American children and adolescents: a randomized controlled trial. Am J Clin Nutr 2013;97:276-285.
Collaborators
- Leslie Biesecker, MD, Genetic Disease Research Branch, NHGRI, Bethesda, MD
- Andrew Butler, PhD, The Scripps Research Institute, La Jolla, CA
- Kong Chen, PhD, Clinical Endocrinology Branch, NIDDK, Bethesda, MD
- Samuel Cushman, PhD, Diabetes Branch, NIDDK, Bethesda, MD
- Myles Faith, PhD, University of Pennsylvania School of Medicine, Philadelphia, PA
- I. Sadaf Farooqi, MD, Cambridge Institute for Medical Research, Cambridge, UK
- Katherine Flegal, MPH, PhD, National Center for Health Statistics, Centers for Disease Control and Prevention, Hyattsville, MD
- Oksana Gavrilova, PhD, Mouse Metabolism Core Laboratory, NIDDK, Bethesda, MD
- Van S. Hubbard, MD, PhD, Division of Nutritional Research Coordination, NIDDK, Bethesda, MD
- Joel E. Kleinman, MD, PhD, Clinical Brain Disorders Branch, NIMH, Bethesda, MD
- Rudolph L. Leibel, MD, Columbia University College of Physicians and Surgeons, New York, NY
- Stephen O'Rahilly, MD, Cambridge Institute for Medical Research, Cambridge, UK
- Dale A. Schoeller, PhD, University of Wisconsin, Madison, WI
- Eric Stice, PhD, Oregon Research Institute, Eugene, OR
- Marian Tanofsky-Kraff, PhD, Uniformed Services University of the Health Sciences, Bethesda, MD
- George R. Uhl, MD, PhD, Molecular Neurobiology Branch, NIDA, Baltimore, MD
- B. Timothy Walsh, PhD, Columbia University College of Physicians and Surgeons, New York, NY
- Heiner Westphal, MD, Program on Genomics of Differentiation, NICHD, Bethesda, MD
- Denise E. Wilfley, PhD, Washington University School of Medicine, St. Louis, MO
- Susan Z. Yanovski, MD, Obesity and Eating Disorders Program, NIDDK, Bethesda, MD
Contact
For further information, contact yanovskj@mail.nih.gov or visit sgo.nichd.nih.gov.