You are here: Home > Section on Genetic Disorders of Drug Metabolism
Molecular Biology, Regulation, and Biochemistry of UDP-Glucuronosyltransferase Isozymes

- Ida S. Owens, PhD, Head, Section on Genetic Disorders of Drug Metabolism
- Nikhil K. Basu, PhD, Staff Scientist
- Sirsendu Jana, PhD, Visiting Fellow
- Amit Raychoudhuri, PhD, Visiting Fellow
- Mousumi Basu, BS, Technician Training Fellow
UDP-glucuronosyltransferase (UGT) isozymes, distributed primarily in the liver, kidney, gastrointestinal tract, and steroid-responsive tissues, carry out the essential function of converting to glucuronides innumerable and frequently encountered structurally diverse lipophilic chemicals that would otherwise be toxic. UGT inactivation of such aromatic-like metabolites and a vast number of dietary, therapeutic, and environmental chemicals reduces the risk of toxicities, mutagenesis and carcinogenesis. Conversion of chemicals to glucuronides inactivates and hastens their excretion from the body, thereby preventing tissue accumulation and toxicities. Neurotoxic bilirubin is the most important endogenous substrate, followed by genotoxic catechol estrogens and dihydrotestosterone at elevated levels. Although the UGT isozyme system prevents bilirubin neurotoxicities in children and inactivates common environmental mutagens and carcinogens, it prematurely converts therapeutics. An understanding of the mechanism of glucuronidation would allow development of methods and strategies for accelerating removal of toxic chemicals while extending the therapeutic benefits of medications that become glucuronidated. In addition to continuing to characterize the individual isozymes that constitute the human UGT chemical defense system, a major research aim of this laboratory has been to understand the basic feature(s)/mechanism(s) underlying the enormous range in substrate selections for UGT isozymes.
Required regulated phosphorylation of the two human prostate-distributed dihydrotestosterone-metabolizing UDP-glucuronosyltransferases supports catalysis and determines cell fate.
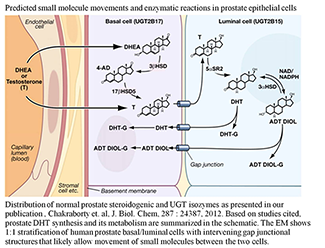
Dihydrotestosterone (DHT) plays a pivotal role in prostate growth, development, and differentiation and is responsible for virilization of male tissues including growth of facial and body hair. Given that insufficient DHT levels during early development lead to defective prostate tissue and that abnormally high DHT levels cause serious virilization syndromes, DHT homeostasis is critically important. During prostate organogenesis, it is essential that the urogenital sinus epithelium receive signals from DHT–occupied androgen receptors located in stromal cells in order to give rise to functional prostate basal and luminal epithelia. In adults gonad-synthesized testosterone is transported to basal cells and the adrenal-synthesized dehydroepiandrosterone (DHEA) steroid precursor is transported via the circulation to prostate basal cells, where appropriate steroidogenic enzymes convert the precursor to testosterone. Subsequently, the testosterone reaches the lumina for 5α-reductase reduction to DHT in order to support androgen-dependent functions.
UGT-2B15:
Human UGT-2B15, which is distributed in the human prostate luminal cell, converts DHT or its 3α-androstane-5α,17β-diol (ADT Diol) metabolite to a glucuronide. As we had previously shown for five other UGTs, UGT-2B15 requires regulated phosphorylation (we had also found that the kinase(s) differ for each UGT isozyme). Reversible downregulation of UGT-2B15–transfected COS-1 following curcumin treatment and irreversible inhibition by three commonly used protein kinase C (PKC) inhibitors versus activation by phorbol 12-myristate 13-acetate (PMA) indicated that UGT-2B15 also undergoes PKC phosphorylation. Mutation of three predicted PKC and two tyrosine kinase sites in UGT-2B15 caused 70–100% and 80–90% inactivation, respectively. PKCα-siRNA treatment inactivated over 50% of COS-1–expressed UGT-2B15. By contrast, we found that treatment of UGT-2B15–transfected COS-1 with the Src-specific activator 1,25 di-hydroxy vitamin D3 enhanced activity, whereas PP2, a specific inhibitor of the tyrosine kinase Src, or Src-siRNA inhibited more than 50% of activity. This and other evidence from our [γ33P]ATP–dependent radiolabelling experiments indicate that UGT-2B15 requires regulated phosphorylation by both PKCα and Src, which is consistent with the complexity of synthesis and metabolism of its major substrate, DHT. Whether basal cells import or synthesize testosterone for transport to luminal cells for reduction to DHT by 5α-steroid reductase-2, luminal-cell UGT-2B15, which exhibits comparatively low-activity, undergoes a complex pattern of regulated phosphorylation necessarily to maintain homeostatic DHT levels to support occupation of the androgen receptor for prostate-specific functions (5).
Figure 1. Predicted small molecule movements and enzymatic reactions in prostate epithelial cells
Image taken from S. K. Chakraborty et al., J Biol Chem 2012;287:24387.
UGT-2B17:
We carried out similar studies to assess whether prostate basal–cell distributed UGT-2B17 also requires regulated phosphorylation, as we had found for UGT-2B15. As described above, regulated phosphorylation is demonstrated by reversible and irreversible inhibition as a result of treatment with curcumin or general PKC inhibitors, respectively. Following UGT-2B17 expression in COS-1 cells and treatment with either PKCε-siRNA, PP2, or Src-siRNA, the enzyme's conversion of both DHT and the androgen metabolite androstanediol (ADT-DIOL) increased, whereas treatment with PKCα-siRNA or PMA downregulated its activity. Such findings suggested downregulation by PKCε/Src and upregulation by PKCα. By contrast, treatment of UGT-2B17 expressed in SrcYesFyn (SYF)−/− cells with Src–inhibitory agents activated UGT-2B17. (This apparent anomaly is also described below in a different and second set of mutational data). For UGT-2B17 phosphorylation–site mutants expressed in COS 1 cells, S172A and S422A were null or unaffected, respectively; Y99F and Y237F mutants revealed selective downregulated DHT glucuronidation over ADT-DIOL by 62% versus 20% and 87% versus 55%, respectively. By contrast, activities for UGT-2B17 mutants expressed in SYF–free cells were similar at around 50% without evidence of substrate preference, and increasing PKCε-siRNA treatment uniquely switched to downregulation, confirming previous upregulation via Src. Co-immunoprecipitation experiments demonstrated that Y99 is a critical UGT-2B17 residue for tyrosine kinase phosphorylation. Moreover, in vitro studies showed that PKCα, PKCε, and Src, individually and additively, phosphorylated UGT-2B17. For prostate-distributed UGT-2B17 and UGT-2B15, which are 95% identical, we showed strong PKCε binding at the modified Src Y99 site in UGT-2B17, binding that downregulates UGT-2B17's activity. Whereas our earlier findings showed that Src alone phosphorylates UGT-2B15, upregulating its luminal-cell activity, evidence presented here indicates that UGT-2B17 is programmed to undergo the opposite Src downregulation (Basu, Basu, Jana, Raychoudhuri, and Owens, manuscript submitted). Furthermore, the co-immunprecipitation results indicated that PKCα phosphorylated S172-UGT-2B17 and S422-UGT-2B17 and that PKCε only failed to phosphorylate when Y99 was mutated.
Because of the complex findings for UGT-2B17 distributed in the prostate basal cell, we also analyzed the phosphorylation requirements for UGT-2B17 by mass spectroscopy. Mass spectrometry confirmed phosphorylation at Y99, S172 , Y237, and S422 and uncovered two sets of overlapping dual phosphorylated residues, (pTpY and pYpS) at 98–99 and 99–100, respectively, in a 4:1 ratio for tryptic fragments of UGT-2B17, with every fragment bi-phosphorylated. Moreover, this finding demonstrates that it is possible for the amino acid triplet TYS at residues 98–100 in UGT-2B17 to exist as a phosphorylated triad, unlike at the corresponding IYG triplet at that position in the 95% identical UGT-2B15 homolog. Whereas treatment of UGT-2B17 expressed in Src–containing cells with Src–specific inhibitors/Src-siRNA was activating, our analysis of UGT-2B17 following expression in Src–/–, Src+/–, and COS-1 cells revealed that UGT-2B17's activity was depressed by 50% by a single Src allele. Hence, the TYS triplet in UGT-2B17 has the capacity to create a critically distinct signaling site that is phosphorylated by both Src and PKCε. Additionally, we substituted the opposing triplet in UGT-2B17 (IYG) and UGT-2B15(TYS) to examine effects on DHT and ADT-DIOL glucuronidation. The results revealed that UGT-2B17(IYG) at 98–100 position had 40% higher activity than wild type and that UGT-2B15(TYS) was 98–100% inactive. Our results demonstrated that TYS is a complex PKε– and Src–binding/phosphorylation/signaling site in UGT-2B17 that depresses glucuronidation (Basu, Basu, Owens, J Biol Chem, manuscript under revision).
Importantly, our studies on the UGT-2B15 and UGT-2B17 isozymes, similar to the unique kinase set for each UGT previously established for four different isozymes, demonstrated that both also require regulated phosphorylation to carry out catalysis by utilizing an energy-dependent non-fixed active-site mechanism, allowing for detoxification of innumerable aromatic chemicals. Unexpectedly, we are now finding that these two prostate-distributed isozymes also undergo phosphorylation in order to participate in and control cell renewal, which is well known to be critical to the health of this organ.
Providing a model for natural events that avert prostate transformation, such phosphorylation studies have allowed us to establish cell-culture conditions that help identify factors controlling and defining the processes that remove a challenged cell and replace it (Basu, Jana, Basu, and Owens; manuscript in preparation).
Comparison of mouse Ugt2b34 and Ugt2b36 isoforms that model human UGT2B7
We extended our studies on human 4-OH-estrone(4-OHE1)/4-OH-estradiol (4-OHE2)–metabolizing UGT-2B7, which detoxifies these depurinating/mutagenizing estrogen metabolites. To identify the mouse Ugt homolog(s) for UGT-2B7 as possible in vivo models, our best-fit computer-based amino acid sequence comparisons identified Ugt-2b34 and Ugt-2b36 isozymes that metabolize 4-OH-estrone with 6- and 30-fold higher km values, respectively, than does UGT-2B7. Moreover, UGT-2B7 metabolizes 4-OH-estrone at a three-fold higher rate than does Ugt-2b34. Despite the 2.7-fold higher turnover rate by Ugt-2b36 compared with UGT-2B7, its 30-fold higher km makes its activity ineffective. Hence, the properties of UGT-2B7 enable it to effectively protect humans against endogenous metabolites. While Ugt-2b34 converts 2-OHE1 at a 30% higher rate than does UGT-2B7, there is no biological evidence that the metabolite is toxic. By contrast, UGT-2B34 and Ugt-2b36 converted the consumer byproduct Bisphenol-A and the medicinal agent diethylstilbestrol, respectively, at robust rates compared with very modest rates by UGT-2B7. Unlike UGT-2B7, which is expressed in human mammary gland, both mouse isozymes are expressed in prostate tissues with very low levels in mammary gland. The findings indicate that mouse prostate is at risk for toxicities by the estrogen metabolites 4-OH-estrone(4-OHE1) and 4-OH-estradiol (4-OHE2).
Inhibition of Ugt-2b34 or Ugt-2b36 expressed in COS-1 cells following treatment with curcumin or with the PKC inhibitor calphostin-C provided evidence that the two mouse isozymes also require regulated phosphorylation that likely supports a non-fixed active site that undergoes phosphorylation signaling as described for the six UGT human isozymes analyzed. Four computer-predicted PKC versus 4-PKC-2 tyrosine kinase sites in Ugt-2b34 and Ugt-2b36, respectively, appear to account for the required regulated phosphorylation.
Whereas all mouse Ugts—Ugt-2b34, Ugt-2b35 and Ugt-2b36—are present at extremely high levels in liver (from 10- to 35-fold higher than that in hormone-responsive tissues), Ugt2b34, which has the critically low km, is uniquely elevated in male hormone–responsive tissues. Prostate has 10%, testis has 2.5%, mammary gland has 1.25% and uterus has traces compared with liver. Ugt-2b36 is present at low but measurable levels in prostate, but it is not in other hormone-responsive tissues. Hence Ugt-2b34 and Ugt-2b36 appear to exhibit activities and tissue distributions that protect against estrogenic metabolites in male hormone-responsive tissues. The evidence suggests that Ugt-2b34 is the designated 4-OHE1–/4-OHE2–metabolizing isozyme in mouse prostate, testis, and mammary gland. Importantly, the findings suggest that the mouse UGT system that protects against reproductive-supporting hormonal agents differs considerably from that of humans. Overall, the evidence from mouse suggests that estrogenic agents are indeed harmful, that mouse prostate is well protected against estrogen metabolites and that this is the basis for the loss or increase in the linked Ugt-2b34 and Ugt-2b36 isozymes following knock-out of the alpha-estrogen receptor or beta estrogen receptor, respectively (which we show appear to be mediated via the estrogen-inducible Pes1 protein, which is also found in the human mammary gland).
Publications
- Basu NK, Kole L, Basu M, Chakraborty K, Mitra PS, Owens IS. The major chemical detoxifying system of UDP-glucuronosyltransferases requires regulated phosphorylation supported by protein kinase C. J Biol Chem 2008;283:23048-23061.
- Banerjee R, Pennington MW, Garza A, Owens IS. Mapping the UDP glucuronic acid binding site in UDP-glucuronosyltransferase-1A10 by homology-based modeling: confirmation with biochemical evidence. Biochemistry 2008;47:7385-7392.
- Mitra PS, Basu NK, Owens IS. Src supports UDP-glucuronosyltransferase-2B7 detoxification of catechol estrogens associated with breast cancer. Biochem Biophys Res Commun 2009;382:651-656.
- Mitra PS, Basu NK, Basu M, Chakraborty S, Saha T, Owens IS. Regulated phosphorylation of a major UDP-glucuronosyltransferase isozyme by tyrosine kinases dictates endogenous substrate selection for detoxification. J Biol Chem 2011;286:1639-1648.
- Chakraborty SK, Basu NK, Jana S, Basu M, Raychoudhuri A, Owens IS. Protein kinase Calpha and Src kinase support human prostate-distributed dihydrotestosterone-metabolizing UDP-glucuronosyltransferase 2B15 activity. J Biol Chem 2012;287:24387-24396.
Collaborators
- Antony McDonagh, PhD, University of California San Francisco, San Francisco, CA
- Masahiko Negishi, PhD, Laboratory of Reproductive and Developmental Toxicology, NIEHS, Research Triangle Park, NC
- Juan Rivera, PhD, Molecular Immunology and Inflammation Branch, NIAMS, Bethesda, MD
- Tapas Saha, PhD, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, D.C.
Contact
For further information, contact ida.owens2@nih.gov.