You are here: Home > Section on Developmental Biology
Molecular Genetics of Embryogenesis in Xenopus and Zebrafish

- Igor B. Dawid, PhD, Head, Section on Developmental Biology
- Reiko Toyama, PhD, Staff Scientist
- Emil Aamar, PhD, Visiting Fellow
- Mi Ha Kim, PhD, Visiting Fellow
- Hyunju Ro, PhD, Research Fellow
- Valeria Zarelli, PhD, Visiting Fellow
- Martha Rebbert, BS, Senior Technician
- John Gonzales, MA, MS, Technician
The laboratory uses the frog Xenopus laevis and the zebrafish Danio rerio as experimental systems to study molecular-genetic mechanisms of early vertebrate development. In the past several years, we have focused on gene discovery by DNA microarray technology and expression profiling in order to identify genes that play a role in the regulation of embryonic development. These approaches have led to studies on mechanisms of gastrulation, neural crest specification, and other patterning processes during embryogenesis.
Organizer restriction through modulation of Bozozok stability by the E3 ubiquitin ligase Lnx-like
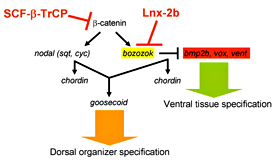
Figure 1. Model for regulatory interactions leading to organizer formation
The stability of β-catenin is regulated by Wnt signaling, which prevents its destruction by SCF-β-TrCP–mediated ubiquitylation. β-catenin induces nodal factors and Bozozok (Boz). Boz executes its function by repressing the ventralizing factors Bmp2b, Vox, Ved, and Vent. Lnx-2b destabilizes Boz, thereby restricting its range and limiting organizer formation to the dorsal region of the embryo. Thus Lnx-2b assures maintenance of balance in the formation of the primary axis of the embryo.
In the vertebrate embryo, the organizer anchors the primary embryonic axis, and balance between dorsal (organizer) and ventral domains is fundamental to body patterning. We have studied a mechanism that regulates the size of the organizer domain by modulating the stability of a key molecule involved in organizer formation. This regulatory pathway is mediated by a member of the Ligand of Numb protein-X (Lnx) family, a RING finger and four PDZ domain containing E3 ubiquitin ligase (see reference 1). LNX proteins have been shown to serve as binding platforms and may play a role in cell fate determination, but their in vivo functions were previously unknown. We showed that Lnx-2b (also known as Lnx-like), a novel member of the family in zebrafish, acts as a critical regulator of dorso-ventral axis formation in this animal (see reference 1). Depletion of Lnx-2b using specific antisense morpholinos (MO) caused strong embryonic dorsalization. We identified Bozozok (Boz; also called Dharma or Nieuwkoid) as a binding partner and substrate of Lnx-2b. Boz is a homeodomain-containing transcriptional repressor induced by canonical Wnt signaling that is critical for the formation of the dorsal organizer. Lnx-2b induced lysine-48–linked polyubiquitylation of Boz, leading to its proteasomal degradation both in human 293T cells and in zebrafish embryos. Dorsalization induced by boz overexpression was suppressed by raising the level of Lnx-2b, but Lnx-2b failed to counteract dorsalization caused by a mutant of boz that lacks a critical motif for Lnx-2b binding. Further, dorsalization induced by depletion of Lnx-2b was alleviated by attenuation of boz expression. We conclude that Lnx-2b modulates Boz activity to prevent the invasion of ventral regions of the embryo by organizer tissue. These studies introduce a ubiquitin ligase, Lnx-2b, as a balancing modulator of axial patterning in the zebrafish embryo (Figure 1). Further, we emphasize the fact that post-translational regulation of protein abundance mediated by the ubiquitin system plays an important role in the establishment of the dorsal-ventral axis during early embryogenesis.
Neural crest formation in Xenopus and zebrafish embryos
We have studied neural crest specification since the time this laboratory discovered the role of Wnt signaling in this process in 1997. Many signaling and transcription factors important in neural crest development have been identified in several laboratories in recent years. Nevertheless, novel components involved in the process are recognized regularly. More recently, we studied three factors involved in early neural crest specification and one factor with a role in subsequent differentiation of neural crest derivatives.
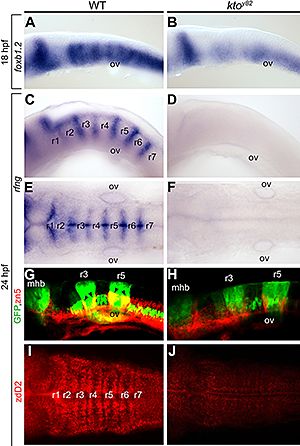
Figure 2. Kctd15 inhibits neural crest induction in Xenopus animal caps.
A. Animal caps can be induced to express the neural crest markers slug, foxd3, and sox9 by injection of chordin (a Bmp antagonist) and Wnt3a. This induction is strongly inhibited by coinjection with Kctd15. B. Stabilized β-catenin (β-cat*), a constitutively active form of the effector of the canonical Wnt pathway, induces neural crest markers even in the presence of excess Kctd15. Odc is used as loading control. (click image to enlarge)
Lrig3, a member of the leucine-rich repeats and immunoglobulin-like domains family, is required for neural crest formation in Xenopus. We showed that Lrig3 modulates Fgf signaling in this process to keep its activity in the optimal range for neural crest formation. Lrig3 may carry out its function by direct interaction with the FGF receptor.
Among Myc family genes, c-Myc is known to play a role in neural crest specification in Xenopus and in craniofacial development in the mouse. We isolated the zebrafish mych (myc homolog) gene, and reported that it is required for neural crest cell survival in this animal.
More recently, we studied the novel factor Kctd15 that restricts neural crest induction (see reference 2). Kctd15 is a BTB domain–containing protein that is first expressed in the embryo in the neural plate border, and subsequently in pharyngeal arches and other regions. Overexpression of Kctd15 strongly inhibits neural crest specification in whole embryos and in animal explants (Figure 2), while attenuation of Kctd15 expression leads to expansion of the neural crest precursor domain. We found that Kctd15 inhibits the output of the canonical Wnt signaling pathway upstream of or at the level of β-catenin (Figure 2). Wnt signaling is known to be crucial for induction of the neural crest and for distinguishing neural crest precursors from precursors of anterior placodes that arise at the neural plate border. We propose that Kctd15 is involved in delineating the separate placode and neural crest domains during embryogenesis (see reference 2).
Neural crest–derived cells make a major contribution to the skeleton of the face. We studied the role of the homeodomain transcription factor Barx1 in the formation of craniofacial cartilages in the zebrafish embryo. We found that the barx1 gene is involved in the regulation of chondrogenesis during pharyngeal arch development. By 2.5 days post fertilization, embryos in which barx1 expression was inhibited show developmental delay exemplified by poor facial outgrowth and micrognathia. Histological analysis revealed a reduction of differentiation and chondrocyte condensation within the arches, and affected larvae exhibit small and dysmorphic arch cartilage elements (Figure 3). The expression of barx1 is controlled by bone morphogenetic protein (BMP) as seen in bead implantation experiments. These results suggest a role for Barx1 at early stages of chondrogenesis within the pharyngeal arches during zebrafish development.
Figure 3. Attenuation of barx1 expression perturbs viscerocranial morphology and cartilage patterning.
Larvae injected with (A,D,G,I) control morpholino (MO) and (B,C,E,F,H,J) Bx MO; Alcian Blue staining (D-H); dissected first and second arches (D-F). (I and J) parasagittal sections of 3 day embryos; (I) control and (J) Bx MO. (A-C) Arrows indicate mouth; arrowheads, pharyngeal arches; (D-F) arrowheads, ceratohyal cartilage; (G, H) asterisk, perturbed fusion; arrowhead, joint fusion between Meckel's cartilage (M) and palatoquadrate (pq); cb, ceratobranchials; ch, ceratohyal; ep, ethmoid plate; hm, hyomandibular; ov, otic vesicle; sy, symplectic element. (click image to enlarge)
The role of Eve1 in formation of caudal nervous tissue in zebrafish
Tail and trunk development in the zebrafish is mediated by several signaling pathways, notably the FGF, BMP, WNT, and retinoic acid (RA) pathways. The role of BMP presents a conundrum for the formation of neural tissue in the posterior domain, given that BMP is widely recognized as a factor that inhibits neurogenesis. In a collaborative study with Tetsuhiro Kudoh, we analyzed the role of the homeodomain transcription factor Eve1, a member of the Evx family, in posterior neural induction in the zebrafish embryo (see reference 3). Eve1 is required for tail formation, in particular the differentiation of posterior neural tissue in the embryo. Eve1 accomplishes its role in two ways: first, Eve1 induces aldh1a2, a gene encoding an enzyme that synthesizes retinoic acid; second, Eve1 attenuates BMP expression, thus permitting the induction of neural tissue. Thus, the role of Eve1 as a key factor in the formation of posterior neural derivatives was explained by its ability to modulate two signaling pathways that control posterior neurogenesis.
Loss of Wif1, a Wnt inhibitory factor, accelerates osteosarcomagenesis.
In pursuit of our interest in the Wnt signaling pathway, we collaborated some years ago in the discovery of a novel Wnt inhibitor named Wif1. The Wnt pathway has numerous roles in development and also contributes to the etiology of many human tumors, as the Wnt signal often promotes cell proliferation and survival. Given the importance of the pathway, we decided to generate a knock-out mouse for Wif1 in which the LacZ gene was inserted into the Wif1 locus, leading to a null mutation. To our surprise and dismay, homozygous mutant mice are viable and fertile. Over the years, several laboratories requested these mice, and eventually the group of David Thomas in Melbourne, Australia, discovered an interesting phenotype of this mutation. They were interested in Wif1 because they found this gene to be silenced epigenetically in many cases of osteosarcoma in humans. Studies using the knock-out mouse model hinted that Wif1 mutant mice develop spontaneous osteosarcomas. More decisively, the formation of radiation-induced osteosarcomas was significantly accelerated in the mutant mice (see reference 4). Thus, the mouse studies support the conclusion that loss of expression of the Wif1 locus is associated with an increased tendency to develop osteosarcomas. The Wif1 mutant mice have been made available to several other laboratories as well, which may yield additional insights into the biological role of this gene.
FGF signaling in zebrafish embryogenesis
The fibroblast growth factor signaling pathway has many important roles in development and adult tissues, and its dysregulation is associated with several human diseases. We studied FGF signaling in zebrafish in several ways, including an inquiry into its involvement in the establishment of left/right asymmetry described in last year's Annual Report. Most recently, we have collaborated with Michael Tsang and his colleagues to delineate the effector molecules that transmit the FGF signal in the zebrafish embryo. Ets-family transcription factors have been implicated in FGF signal transduction in other systems, and we find that three Ets factors in the Pea3 subfamily are the primary effectors of FGF signaling in embryogenesis, specifically in cardiac development and in the establishment of left/right asymmetry (see reference 5). The three Pea3 factors function in a partly redundant manner so that interference with the expression of all three factors largely mimics the loss of FGF signaling in the embryo.
Publications
- Ro H, Dawid IB. Organizer restriction through modulation of Bozozok stability by the E3 ubiquitin ligase Lnx-like. Nat Cell Biol. 2009;11:1121-1127.
- Dutta S, Dawid IB. Kctd15 inhibits neural crest formation by attenuating Wnt/β-catenin signaling output. Development 2010;137:3013-3018.
- Cruz C, Maegawa S, Weinberg ES, Wilson SW, Dawid IB, Kudoh T. Induction and patterning of trunk and tail neural ectoderm by the homeobox gene eve1 in zebrafish embryos. Proc Natl Acad Sci U S A 2010;107:3564-3569.
- Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M, Dobrovic A, Slavin J, Choong PF, Simmons PJ, Dawid IB, Thomas DM. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest. 2009;119:837-851.
- Znosko WA, Yu S, Thomas K, Molina GA, Li C, Tsang W, Dawid IB, Moon AM, Tsang M. Overlapping functions of Pea3 ETS transcription factors in FGF signaling during zebrafish development. Dev Biol. 2010;342:11-25.
Collaborators
- Neil A. Hukriede, PhD, University of Pittsburgh, Pittsburgh, PA
- Tetsuhiro Kudoh, PhD, Exeter University, Exeter, UK
- Kosuke Tanegashima, PhD, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan
- David M. Thomas, MD, Peter MacCallum Cancer Centre, Melbourne, Australia
- Michael Tsang, PhD, University of Pittsburgh, Pittsburgh, PA
- Hui Zhao, PhD, The Chinese University, Hong Kong, China
Contact
For more information, email dawidi@mail.nih.gov or visit sdb.nichd.nih.gov.