You are here: Home > Section on Human Genetics
The Chromatin-Based Epigenome in Development and Aging

- Bruce H. Howard, MD, Head, Section on Human Genetics
- Valya Russanova, PhD, Staff Scientist
- Alice Berg, BS, Postbaccalaureate Fellow
- Paraskevi Salpea, BS, Predoctoral Fellow
The normal human lifespan is marked by a complex series of developmental transitions, with relative stability during adulthood and, ultimately, a gradual decline in viability. Clock-like mechanisms presumably underlie the developmental events that occur through childhood and adolescence. Further, instabilities in such mechanisms are likely to be an integral part of the aging process and to contribute to many common degenerative diseases of later life. A breakthrough in the area of developmental transitions would have remarkable implications; thus, there is widespread interest in the prospect that epigenetics—and the rapidly evolving field of epigenomics—holds the key to further progress.
Whole genome comparisons of epigenome structure
The past year has seen the continuing implementation of high-throughput genomics approaches in studies that use human tissues from several clinical sources. We obtain peripheral blood monocytes from newborns (cord blood) through a collaboration with the Perinatology Branch, NICHD, while monocytes from adults are available through the NIH Department of Transfusion Medicine and the Mid-Atlantic Twin Registry (MATR). These cells are induced to differentiate in vitro into antigen-presenting dendritic cells. We procure human skin fibroblasts from newborns and adults, as needed, under a protocol approved by the NICHD Institutional Review Board. We induce the latter cells to enter a quiescent state by serum deprivation for six or more days and then examine them with respect to gene expression and chromatin structure upon serum stimulation.
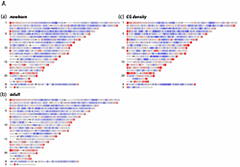
Whole genome comparison of DNA methylation densities
DNA methylation densities were compared for human newborn and adult genomes by MeDIP. Key: red, greater or equal to 2-fold over-representation; blue, less than or equal to 2-fold under-representation relative to input control. Resolution 2 mb. (click image to enlarge)
Using these monocyte- and fibroblast-based experimental systems, we previously studied developmental and age-related changes in gene regulation. Our current work employs next-generation (next-Gen) sequencing-based whole-genome surveys to explore the mechanism(s) underlying such changes. In mammalian cells, cis-dependent epigenetic states are maintained by both chromatin structure and DNA methylation. Chromatin states are measured with respect to histone acetylation and methylation patterns, as well as the topologies of these patterns extending over domains that typically encompass one or more genes. With the advent of next-Gen sequencing, DNA methylation can now be characterized with nucleotide resolution over these gene-size domains. Of particular interest to us are interactions between the chromatin- and DNA methylation–based components of epigenetic control.
Given the large data sets that are generated by chromatin immunoprecipitation (ChIP) studies—both using microarray-based ChIP-on-chip and next-Gen sequencing–based (ChIP-seq) analyses—appropriate bioinformatics tools are essential. Along these lines, we have developed genome annotation, pattern recognition, and pattern comparison algorithms. For high-resolution mapping of DNA methylation patterns, we also designed software to resolve the alignment problem associated with sequence analysis of bisulfite-modified DNA. Further, new bioinformatic tools have been developed to more efficiently link patterns in RNA expression data sets with epigenome features.
Chromatin-related results to date indicate that genes subject to both differentiation and developmental controls depend on the three-dimensional topology of the genome and are sensitive to the remodeling of higher-order chromatin structures. We will continue to focus on large (on the order of 100 kb) domains over which histone acetylation or histone H3-K27 patterns are altered, as well as on control elements that may assume non–B DNA conformations. DNA methylation–related results suggest that a little-studied subcompartment of the genome comprising tandem arrays and gene clusters may be highly enriched for developmental- and age-related epigenome remodeling.
The emerging goal is to generalize our paradigm to address a range of current problems in maternal reproductive health, pediatrics, and areas of medicine relating to age-related disease. Based on the genes currently under study, we will most likely investigate deficiencies in the innate immune systems of newborns, peripheral insulin resistance and diabetes in adolescents and young adults, and a spectrum of neurodegenerative processes, including Parkinson's and Alzheimer's diseases, in the elderly.
Publications
- Humphrey GW, Wang YH, Hirai T, Padmanabhan R, Panchision DM, Newell LF, McKay RDG, Howard BH. Complementary roles for histone deacetylases 1, 2 and 3 in differentiation of pluripotent stem cells. Differentiation 2007;76:348-356.
- Porrás A, Kozar S, Russanova V, Salpea P, Hirai T, Sammons N, Mittal P, Kim JY, Ozato K, Romero R, Howard BH. Developmental and epigenetic regulation of the human TLR3 gene. Mol Immunol 2008;46:27-36.
Collaborators
- Jonathan Epstein, MS, Scientific Software Support and Bioinformatics Core Facility, NICHD, Bethesda, MD
- Roberto Romero, MD, Program in Perinatal Research and Obstetrics, NICHD, Detroit, MI
Contact
For more information, email howard@helix.nih.gov.


