You are here: Home > Section on Formation of RNA
Physiological, Biochemical, and Molecular Genetic Events of Recognition and Resolution of RNA/DNA Hybrids

- Robert J. Crouch, PhD, Head, Section on Formation of RNA
- Susana M. Cerritelli, PhD, Staff Scientist
- Hyongi Chon, PhD, Postdoctoral Fellow
- Lina Gugliotti, PhD, Postdoctoral Fellow
- John B. Holmes, BS, Predoctoral Fellow, NIH/Oxford/Cambridge Graduate Partnerships Program
- Kiran Sakhuja, MS, MSc, Research Assistant
- Yutaka Suzuki, PhD, Postdoctoral Fellow
Damaged DNA is one of the leading causes of many human diseases and disorders. Our studies are directed toward the formation and resolution of RNA/DNA hybrids, which occur during DNA and RNA synthesis. Such hybrid molecules may lead to increased DNA damage but may also play critical roles in normal cellular processes. We are interested in understanding how RNA/DNA hybrids are resolved and the role that ribonucleases H (RNases H) play in their elimination. Two classes of RNases H are present in most organisms. Our studies have shown that mice deleted for the Rnaseh1 gene arrest embryonic development at day 10 as a result of failure to amplify mitochondrial DNA. Others have found that the Aicardi-Goutières Syndrome (AGS), a severe neurological disorder with symptoms appearing at or soon after birth, can be caused by defective human RNase H2. We employ molecular-genetic and biochemical tools and yeast and mouse models in our research.
RNA/DNA hybrids can be composed of one strand of RNA and a second strand of DNA or can be in the form of an R-loop in which the two DNA strands are separated, with only one hybridized to RNA while the other is single-stranded DNA. Both types of hybrid are substrates for the two classes of RNases H. Simple (non-R-loop) RNA/DNA hybrids are formed when the HIV-AIDS virus copies its genomic RNA into DNA using a reverse transcriptase (RT). The RT also has an RNase H domain that is structurally and functionally similar to the class I cellular RNase H and is necessary in several steps of viral DNA synthesis. It is known that during RNA synthesis R-loops can form and that aberrant R-loop formation can result in chromosome breakage. However, R-loop formation has been observed in the normal recombination process of switching (recombination) from one form of immunoglobulin to another, resulting in different isoforms of antibodies.
Structure-function of ribonucleases H: RNase H1
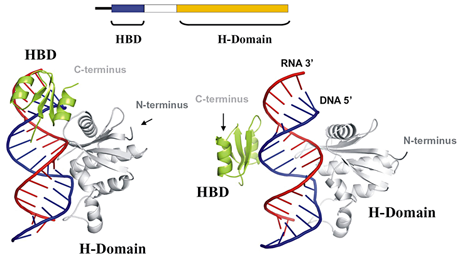
Figure 1. Organization and structures of RNase H1 in complex with RNA/DNA hybrid
A simple image of the linear organization of human RNase H1 is shown together with two views of RNase H1 interacting with and RNA/DNA hybrid. The views show two different relative positions of the HBD and RNase H domain. The region connecting the two domains is flexible, with the C-terminus of the HBD positioned 25 (left) or 60 (right) Å from the N-terminus of the RNase H domain. (click image to enlarge)
RNA/DNA hybrids are essential intermediates in the replication of HIV's RNA genome. In addition, the hybrids are believed to be necessary for mitochondrial DNA replication and important for switching immunoglobulin isotypes (e.g., from IgM to IgA). How these enzymes recognize and cleave the RNA is important to an understanding of the biology of these diverse events and for possible regulation of RNase H activity. Bacterial RNases HI are generally small proteins (150 amino acids) that share significant similarity with their eukaryotic counterparts in structure, interaction with their substrates, and the mechanism of cleavage. The protein structure of E. coli RNase HI unbound to a substrate is similar to that of the structure of human RNase H1 in complex with an RNA/DNA hybrid. The enzyme recognizes at least four hydroxyl groups of the ribose, with the DNA significantly distorted so that one phosphate is able to bind in a pocket on the enzyme (Nowotny et al., Cell 2005;121:1005). Both properties contribute to the specificities of the enzyme. The "basic protrusion" of the enzyme has extensive interactions with the hybrid, adding to the stability of the complex. Eukaryotic RNases H have an N-terminal domain (absent from the bacterial enzyme) that binds to RNA/DNA, conferring processivity to the enzyme (Gaidamakov et al., Nucleic Acids Res 2005;33:2166). This N-terminal domain (HBD) interacts with RNA/DNA through contacts with both the DNA and RNA strands and may provide the initial contact between the enzyme and hybrid. (See Figure 1 for organization and interactions of RNase H1 with RNA/DNA hybrids.)
The N- and C-terminal domains are connected by 65 amino acids in the human enzyme and by 64 in the mouse enzyme. The length and sequence of the connection domain are extremely variable in other species, suggesting that RNase H activity may not depend on any particular amino acid number or sequence. Our studies indicate that the connection domain is important for flexibility, allowing the protein to bind and cleave more effectively. The cleavage events are, first, binding of the HBD to the substrate, whereafter the RNase H domain searches for an appropriate cleavage site and cleaves; following release of the RNase H domain from the hydrolyzed substrate, the HBD anchors the protein while the RNase H domain recognizes another site and cleaves; the process continues until either the HBD releases from the hybrid or no more RNase H cleavage sites are available to be attacked.
Functions of RNase H1 in mitochondria and nuclei
One of the major challenges we face in understanding the roles of RNase H1 in cells is to determine how the translation of a single mRNA can produce both nuclear and mitochondrial forms of the enzyme. Knocking out the Rnaseh1 gene in mouse results in embryonic lethality at embryonic day 8.5 as a result of a failure to replicate mitochondrial DNA. The outcome indicates that 1) the enzyme is essential for the maintenance of mtDNA and 2) there is no need for newly synthesized RNase H1 for replication/repair of nuclear DNA. Using antibodies that bind to RNA/DNA hybrids, we showed that extensive amounts of hybrids exist during replication of mtDNA. How these RNA/DNA hybrids remain in the presence of the mitochondrial form of RNase H1 is unclear. However, RNase H1 is involved in hybrid maintenance and possibly in converting RNAs of hybrids for use as RNA primers for DNA synthesis. Expression of an excess of the mitochondrial form of RNase H1 in HEK293 cells bearing either the mouse of human Rnaseh1 gene results in loss of mtDNA and cell death. Neither mtDNA loss or cell death occurs when only the nuclear form of RNase H1 is highly expressed.
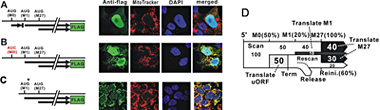
Figure 2. Translational regulation of RNase H1
A: Localization of of RNase H1: when three AUG codons at M0-M1 and M27 are present, RNase H1 is mainly present in the nucleus, with only very minor amounts co-localizing with the red mitochondrial signal.
B: Increase of mitochondrial localization when M0-AUG is absent.
C: Improving the context of AUG at M1 increases the amount of Rnase H1 in mitochondria.
D: A model of leaky scanning during translation of Rnaseh1 mRNA.
(click image to enlarge)
Translation of the single Rnaseh1 mRNA initiates at two distinct start codons, with the resulting proteins being targeted to either the mitochondria or the nucleus (see Figure 2). Our current studies demonstrate that the level of each protein is affected by a short upstream open reading frame. Thus, the amount of protein(s) is (are) kept at low levels by differential translation. The organization of the uORF M1 and M27 translation start sites of RNases H1 is conserved from flies to humans, underscoring the importance of regulating the amount of RNase H1 in mitochondria and nuclei. When ribosomes interact with the Rnaseh1 mRNA, they scan from the 5'-end of the mRNA, first encountering the AUG codon (M0) and either initiate synthesis of a short peptide (uORF) or move on to the second AUG (M27). If protein synthesis starts at M27, the mitochondrial targeting sequence (MTS) is included in the product and the protein is sent to mitochondria. If the first AUG for the start of RNase H1 synthesis is at M27, only the nuclear isoform of the enzyme is produced. The presence of M0 reduces the amounts of both the mitochondrial and nuclear RNases H1 but that of the mitochondrial form to a greater extent (compare the amounts of RNase H1 in the mitochondria in Figure 2A and 2B). If the context of the AUG at M0 is improved for more efficient initiation, the level of protein in mitochondria increases significantly (Figure 2C). The model of translational efficiencies determined experimentally is shown in Figure 2D.
This finding suggests that the very low amount of Rnaseh1 mRNA observed in most cells is more than sufficient to generate the optimal quantity of RNase H1 and that relatively poor translation is used to limit the amount of enzyme produced. We have generated transgenic mice that express higher levels of RNase H1 in B cells, the increase resulting in no obvious change in mitochondria. We are examining effects of even higher levels of the nuclear form of RNase H1 in B cells, in which recombination of the immunoglobulin locus results first in joining of the VDJ regions; then later in B cell development the isotype switching occurs. These events involve numerous proteins used during repair of damaged DNA.
We have generated conditional knockout mice that permit us to determine whether RNase H1 is necessary in adult animals either for mtDNA or nuclear DNA replication and repair. It may be that RNase H1 is important for mtDNA during embryogenesis only when, after implantation, rapid mtDNA synthesis occurs, at which time the enzyme is suddenly activated. In addition, we will be able to generate organ-/tissue-specific knockouts of the Rnaseh1 gene by using the Cre-lox system, including tamoxifen-inducible Cres for general ablation and for others (e.g., heart-specific Cre expressers). However, the problem of inactivating the synthesis of both forms remains unresolved. Accordingly, we have generated transgenic mice that produce either both isoforms of RNase H1 or only the nuclear form in T and B cells.
In collaboration with Ian Holt, we are searching for possible roles of RNase H1 in mitochondrial DNA replication by using, among other techniques, analysis of intermediates on two-dimensional gels. Our findings thus far indicate that elevated expression of RNase H1 in mitochondria alters mtDNA replication. In addition, data obtained in Holt's laboratory by John Holmes supporting the existence of RNA/DNA hybrids as replication intermediates indicate a bi-directional mode of DNA replication for this organelle.
Structure-function of ribonucleases: RNase H2
We previously found that, in Saccharomyces cerevisiae, RNase H2, the second type of RNase H, is composed of three subunits (Jeong et al., Nucleic Acids Res 2004;32:407), two of which we did not find in higher eukaryotes when using a BLAST search. We determined that there are three subunits of RNase H1 in human cells and were able to find similar RNases H2 in other mammals, in particular in the mouse. A recent paper in Nature Genetics reported that mutations in any one of these proteins leads to the rare Aicardi-Goutières syndrome (AGS). Our studies on the human and yeast enzymes indicate a connection between RNase H2 and PCNA (proliferating cell nuclear antigen)—a clamp-loader protein involved in recruiting proteins to DNA for repair and replication. A PCNA-interacting peptide (PIP) is present in the RNase H2B subunit of yeast, mouse, and human RNases H2. Using several types of analyses, we demonstrated that the PIP of the RNase H2B is indeed able to interact with PCNA, a finding whose biological significance we are continuing to explore. We also examined the effects of several of the AGS-causing mutations on recombinant RNase H2 activity. Only two of the mutant enzymes have shown decreased activity. The other altered proteins are most likely defective in vivo for complex formation and/or stability.
Publications
- Nowotny M, Cerritelli SM, Ghirlando R, Gaidamakov SA, Crouch RJ, Yang W. Specific recognition of RNA/DNA hybrid and enhancement of human RNase H1 activity by HBD. EMBO J 2008 27:1172-1181.
- Chon H, Vassilev A, DePamphilis ML, Zhao Y, Zhang J, Burgers PM, Crouch RJ, Cerritelli SM. Contributions of the two accesory subunits, RNASEH2B and RNASEH2C, to the activity and properties of the human RNase H2 complex. Nucleic Acids Res. 2009 37:96-110.
- Cerritelli SM, Crouch RJ. Ribonuclease H: the enzymes from eukaryotes. FEBS J 2009 276:1494-1505.
- Pohjoismäki JLO, Holmes JB, Wood SR,Yang M-Y, Yasukawa T, Reyes A, Bailey LJ, Cluett TJ, Goffart S, Willcox S, Rachel E, Rigby RE, Jackson AP, Spelbrink JN, Griffith JD, Crouch RJ, Jacobs HT, Holt IJ. Mammalian mitochondrial DNA replication intermediates are essentially duplex but contain extensive tracts of RNA/DNA Hybrid. J Mol Biol. 2010 397:1144-1155.
- Suzuki Y, Holmes JB, Cerritelli SM, Sakhuja K, Minczuk M, Holt IJ, Crouch RJ. An upstream open reading frame and context of the two AUG codons affect the abundance of mitochondrial and nuclear RNase H1. Mol Cell Biol. 2010; in press.
Collaborators
- Peter Burgers, PhD, Washington University, St. Louis, MO
- Ian Holt, PhD, MRC-Dunn Nutrition Unit, Cambridge, UK
- Shigenori Kanaya, PhD, Osaka University, Osaka, Japan
- Paul E. Love, MD, PhD, Program on Genomics of Differentiation, NICHD, Bethesda, MD
- Herbert C. Morse, MD, Laboratory of Immunopathology, NIAID, Bethesda, MD
- Marcin Nowotny, PhD, International Institute of Molecular and Cell Biology, Warsaw, Poland
- Wei Yang, PhD, Laboratory of Molecular Biology, NIDDK, Bethesda, MD
Contact
For more information, email crouch@helix.nih.gov or visit sfr.nichd.nih.gov.


