You are here: Home > Unit on Behavioral Neurogenetics
Neuronal Circuits Controlling Behavior: Genetic Analysis in Zebrafish

- Harold Burgess, PhD, Head, Unit on Behavioral Neurogenetics
- Sadie A. Bergeron, PhD, Postdoctoral Fellow
- Kandice Fero, PhD, Postdoctoral Fellow
- Kathryn M. Tabor, PhD, Postdoctoral Fellow
- Tohei Yokogawa, PhD, Postdoctoral Fellow
- Diana Jordan, BSc, Technical Intramural Research Training Award Trainee
- Jennifer L. Strykowski, MSc, Zebrafish Technician
Our goal is to understand how neuronal circuits in larval zebrafish produce appropriate motor responses under diverse environmental contexts. Locomotor behavior in zebrafish larvae is controlled by neuronal circuits that are established through genetic interactions during development. We aim to identify genes and neurons that are required for the construction and function of the brainstem circuits underlying specific behaviors. In vertebrates, neuronal circuits situated in the brainstem form the core of the locomotor control network and are responsible for balance, posture, motor control, and arousal. Accordingly, many neurological disorders stem from abnormal formation or function of brainstem circuits. Insights into the function of brainstem circuits in health and disease have come from genetic manipulation of neurons in zebrafish larvae in combination with computational analysis of behavior.
Several unique features of the zebrafish model system facilitate analysis of the neuronal basis of vertebrate behavior. The larval zebrafish brain exhibits the basic architecture of the vertebrate brain but is much less complex than the mammalian brain. The optical clarity of the embryo facilitates visualization of individual neurons and their manipulation with genetic techniques. Behavior in larvae is innate and therefore exhibits minimal variability between fish. Subtle alterations in behavior can therefore be robustly scored, making it possible to assess quickly the contribution of identified neurons to a variety of motor behaviors. We use two major behavioral paradigms to investigate the neuronal basis of vertebrate behavior: modulation of the acoustic startle response by prepulse inhibition and modulation of the locomotor repertoire during a phototaxis-based navigational task. In addition, we are developing a suite of genetic tools and behavioral assays to probe the nexus between neuronal function and behavior at single-cell resolution.
Neuronal circuitry underlying arousal states in zebrafish
Click image to enlarge.
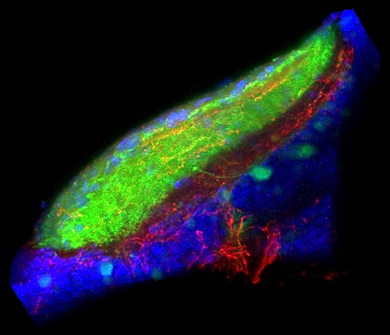
Figure 1. Sensory-motivational integration in the optic tectum
Our results implicate the otic tectum (the zebrafish homolog of the superior colliculus) as a site where motivational information modulates sensory input. Retinal ganglion fibers (green) convey visual information to the tectum, where they overlap with fibers from dorsal raphe neurons, which signal arousal (red). Cell nuclei counterstained blue.
Many neurological conditions include impairments in arousal, but the complexity of the neuronal systems subserving arousal in mammals has made it difficult to understand precisely how various neuromodulator systems contribute to its control. We set out to establish a robust system for inducing arousal in zebrafish larvae. One hallmark of arousal states is elevated motor activity that persists beyond the stimulus inducing the arousal state. We speculated that the visual motor response—hyperactivity triggered by sudden darkness—might represent an arousal state in zebrafish. While performing a detailed behavioral characterization of this response, we found that the visual behavior did not require classical light-sensing organs: the eyes and pineal. Further investigation demonstrated that the behavior is triggered by deep-brain photoreceptors, the melanopsin-expressing neurons in the preoptic hypothalamus (1).
In a second approach to investigating arousal, we studied the function of the serotonergic system. The serotonergic system is a major neurotransmitter system modulating arousal in higher vertebrates. To ascertain the role of this system in behavioral control in larval zebrafish, we generated transgenic fish expressing the Gal4 transcription factor in serotonergic neurons. We can thus genetically manipulate the neurons by crossing the fish to lines carrying reporter genes under the control of the UAS (upstream activating sequence) promoter. We found that exposure to water flow induces a short-term state of arousal characterized by increased locomotor activity and sensitivity to an optomotor stimulus. Ablation of the serotonergic neurons of the dorsal raphe impairs sensory responsiveness during flow-induced arousal. By injecting the Gal4 transgenic fish with a UAS:GCaMP (GCaMP is a genetically encoded calcium indicator) element, we performed in vivo calcium imaging of serotonergic neurons and found that subsets of dorsal raphe neurons are active during flow-induced arousal. High-resolution neuronal tracing, using genetic mosaic techniques, demonstrated that neurons of the dorsal raphe project to the optic tectum, a likely site for the modulation of sensory responsiveness to visual cues (2). We are continuing to use a combination of approaches to correlate the connections of single serotonergic neurons with their behavioral role, establishing a neuron-level functional map of serotonergic anatomy.
We are currently characterizing a distinct short-term arousal-like state induced in zebrafish larvae through exposure to heat. Our data indicate that distinct populations of neurons in the trigeminal ganglion trigger fast startle responses to sudden intense heat and prolonged arousal states in response to lower levels of heat exposure.
Modulation of the acoustic startle response
Zebrafish larvae show a robust escape response to sudden stimuli in a variety of modalities. We noted that escape responses also occur in response to a brief electric field pulse (EFP). Our analysis showed that EFP responses are well coordinated swimming movements that share circuitry with the acoustic startle response, as they are mediated by the same central command neurons, the Mauthner cells. This ongoing work has revealed a surprising new modality for triggering startle responses in zebrafish. Previously, we demonstrated that the startle responses in zebrafish larvae are modulated in a similar fashion to startle responses in mammals, in which startle magnitude is inhibited when the startle stimulus is preceded by a weak auditory prepulse. This form of startle modulation, termed prepulse inhibition, is diminished in several neurological conditions, including schizophrenia. To identify neurons involved in prepulse inhibition, we conducted a circuit-breaking screen, genetically ablating neurons labeled in enhancer trap lines to identify neurons that modulate startle responses. We generated these lines using a novel method that enriches for neuron-specific transgene expression (4). Our circuit-breaking screen enabled us identify a transgenic line that labels a discrete population of neurons in the hindbrain whose ablation or optogenetic inhibition leads to dysregulation of prepulse inhibition and which connect to the Mauthner cells (Figure 2). Recently, we extended our work in zebrafish to show that similar neurons are likely to modulate the startle responsiveness in mammals. As prepulse inhibition is abnormal in neuro-psychiatric disorders with developmental origins, including schizophrenia and autism, our work will help identify fundamental defects in circuitry abnormal in these conditions.
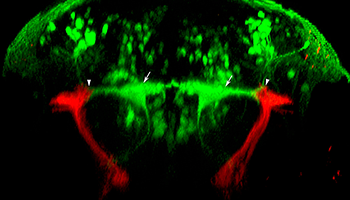
Figure 2. Sensorimotor interface at the lateral dendrite of the Mauthner cell
We generated a library of Gal4 enhancer trap lines including y256, which labels stato-acoustic ganglion neurons. In this transverse image through the hindbrain of a larva, y256 drives expression of a membrane-tagged red fluorescent protein, allowing tracing of their projections into the brain. Stato-acoustic ganglion neurons project dorsally and contact the bilateral pair of Mauthner cells (green, arrows), the command neurons for the startle response, at the distal tips of their lateral dendrites (arrowheads).
Tools for analyzing neuronal circuits that control behavior
The relatively simple nervous system of zebrafish larvae and the larva's restricted range of motor behaviors make it possible to identify neuronal pathways that underlie the entire behavioral repertoire. However, it would be extremely useful to have reporter lines that would permit the manipulation of small groups of neurons known to be involved in a particular motor behavior. Many existing transgenic lines labeling neurons do exist, but most show expression outside the brain, limiting the usefulness of these lines for behavioral analysis in unrestrained fish. We developed a technique to restrict reporter gene expression to the CNS by adapting the neuron-restrictive silencing element (NRSE/RE1), then performing an enhancer trap screen, thus creating a library of 240 Gal4 enhancer-trap transgenic fish with unique and restricted patterns of neuronal expression. The lines constitute a unique resource for decoding the developmental genetics and neuro-anatomical basis of behavior in zebrafish larvae. We have now mapped the integration site of the transgene in many of these lines. In around 15% of lines, the integration is in an exon or early intron of a gene, potentially disrupting protein expression. Indeed, in one such line, y241 larvae with two copies of the transgene show a pronounced behavioral defect, with greatly reduced motility from the earliest stages of development. In addition we generated new tools that allow the Gal4 lines to be used to manipulate neuronal circuitry in vivo. Recently we developed a significantly more active version of the bacterial nitroreductase gene used for cellular ablation. To complement this work, we also developed reporter lines allowing neurons to be sensitized. Together, these methods enable us to interrogate neuronal cell function in vivo and assess their contribution to behavior.
Publications
- Fernandes AM, Fero K, Arrenberg AB, Bergeron SA, Driever W, Burgess HA. Deep brain photoreceptors control light seeking behavior in zebrafish larvae. Curr Biol 2012;22:1-6.
- Yokogawa T, Hannan MC, Burgess HA. The dorsal raphe modulates sensory responsiveness during arousal in zebrafish. J Neurosci 2012;32:15205-15215.
- Ikeda H, Delargy A, Yokogawa T, Urban JM, Burgess HA, Ono F. Neuronal activity under ethanol-clamp in zebrafish revealed resistance to ethanol. PLoS One 2013;8:e63318.
- Bergeron SA, Hannan MC, Codore H, Fero K, Li G, Moak Z, Yokogawa T, Burgess HA. Brain selective transgene expression in zebrafish using an NRSE derived motif. Front Neural Circuits 2012;6:110.
- Fernandes AM, Fero K, Driever W, Burgess HA. Enlightening the brain: linking deep brain photoreception with behavior and physiology. BioEssays 2013;35:775-779.
Collaborators
- James J. Dowling, MD, PhD, University of Toronto, Toronto, Canada
- Wolfgang Driever, PhD, Universität Freiburg, Freiburg, Germany
- Michael J. Iadarola, PhD, Warren Grant Magnuson Clinical Center, NIH, Bethesda, MD
- Ralph Nelson, PhD, Basic Neurosciences Program, NINDS, Rockville, MD
- Fumihito Ono, PhD, Laboratory of Molecular Physiology, NIAAA, Rockville, MD
Contact
For more information, email haroldburgess@mail.nih.gov or visit ubn.nichd.nih.gov.