You are here: Home > Unit on Behavioral Neurogenetics
Neuronal Circuits Controlling Behavior: Genetic Analysis in Zebrafish

- Harold Burgess, PhD, Head, Unit on Behavioral Neurogenetics
- Sadie A. Bergeron, PhD, Postdoctoral Fellow
- Eric J. Horstick, PhD, Postdoctoral Fellow
- Kathryn M. Tabor, PhD, Postdoctoral Fellow
- Tohei Yokogawa, PhD, Postdoctoral Fellow
- Diana Jordan, BSc, Technical Intramural Research Training Award Trainee
- Mary R. Brown, BSc, Postbaccalaureate Fellow
- Jennifer L. Strykowski, MSc, Zebrafish Technician
Our goal is to understand how neuronal circuits in larval zebrafish produce appropriate motor responses under diverse environmental contexts. Locomotor behavior in zebrafish larvae is controlled by neuronal circuits that are established through genetic interactions during development. We aim to identify genes and neurons that are required for the construction and function of the brainstem circuits underlying specific behaviors. In vertebrates, neuronal circuits situated in the brainstem form the core of the locomotor control network and are responsible for balance, posture, motor control, and arousal. Accordingly, many neurological disorders stem from abnormal formation or function of brainstem circuits. Insights into the function of brainstem circuits in health and disease have come from genetic manipulation of neurons in zebrafish larvae in combination with computational analysis of behavior.
Several unique features of the zebrafish model system facilitate analysis of the neuronal basis of vertebrate behavior. The larval zebrafish brain exhibits the basic architecture of the vertebrate brain but is much less complex than the mammalian brain. The optical clarity of the embryo facilitates visualization of individual neurons and their manipulation with genetic techniques. Behavior in larvae is innate and therefore exhibits minimal variability between fish. Subtle alterations in behavior can therefore be robustly scored, making it possible to assess quickly the contribution of identified neurons to a variety of motor behaviors. We focus on two aspects of behavioral regulation: the mechanisms by which sensory context regulates behavioral decisions; and pathways that sustain changes in behavioral state. In addition, we are developing a suite of genetic tools and behavioral assays to probe the nexus between neuronal function and behavior at single-cell resolution.
Molecular identification of neurons that mediate prepulse inhibition
Click image to enlarge.
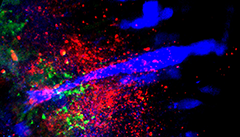
Figure 1. Connections between Gsx1 neurons and the Mauthner cell
Confocal section showing the Mauthner cell (blue), synapses formed by Gsx1 neurons (red), and the location of glutamatergic synapses (green).
In mammals, including humans, the startle response to a strong auditory stimulus can be inhibited by pre-exposure to a weak acoustic 'prepulse.' This form of startle modulation, termed prepulse inhibition, is diminished in several neurological conditions, including schizophrenia. The precise pattern of neuronal connections that enable prepulse inhibition is not known, yet understanding the mechanism of prepulse inhibition would enable rational investigation of how schizophrenia risk factors influence neuronal circuitry. Vibrational stimuli trigger rapid-escape swims in zebrafish that are mediated by giant reticulospinal neurons similar to the central neurons controlling startle responses in mammals. Escape swims are suppressed by pre-exposure to a prepulse, allowing us to apply the powerful suite of genetic tools in zebrafish to identify neurons that mediate prepulse inhibition.
To identify a transgenic zebrafish line that genetically labels neurons required for prepulse inhibition, we conducted a circuit-breaking screen. In this screen, we first generated a library of neuron-specific Gal4–enhancer trap lines marking distinct populations of neurons in the brain, then ablated the neurons in each enhancer trap line before testing for prepulse inhibition. We identified a transgenic line, y252, that labeled a discrete population of neurons in the hindbrain whose ablation or optogenetic inhibition led to dysregulation of prepulse inhibition. Dorsally located in the hindbrain, y252 neurons sent ventral projections toward the lateral dendrite of the Mauthner cell, the command neuron for escape responses in fish. We analyzed expression of neurotransmitter markers in y252 neurons and found that the neurons are located within one of four longitudinal columns of glutamatergic neurons extending through the rostro-caudal axis of the hindbrain (Figure 1). Moreover, blockade of NMDA receptors with MK801 phenocopied the effect of y252 neuron ablation, confirming that glutamatergic signaling is a central part of the mechanism for prepulse inhibition.
Next, we discovered that the neurons genetically labeled in y252 are specified by Gsx1, a transcription factor previously implicated in differentiation of both excitatory and inhibitory interneurons in the spinal cord. Gsx1 was expressed in the mouse brainstem in the proliferative zone that gives rise to regions previously implicated in prepulse inhibition. We obtained gsx1 knockout mice and performed behavioral testing. Knockout mice showed normal startle sensitivity but a strong reduction in prepulse inhibition. The results thus show that Gsx1 expression defines neurons that are required for prepulse inhibition across vertebrate species (1). As prepulse inhibition is abnormal in neuropsychiatric disorders with developmental origins, including schizophrenia and autism, our work will help identify and probe fundamental defects in circuitry abnormal in these conditions.
Analysis of neuronal circuitry underlying electric field pulse responses
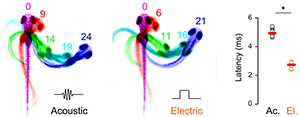
To dissect prepulse inhibition circuits in fish, we tested other sensory stimuli that elicit escape swims. We found that well coordinated escape responses also occur in response to a brief electric field pulse (EFP). The reaction time for EFP responses was remarkably short, leading us to question whether they were mediated by peripheral sensory neurons, as we had suspected (Figure 2). To address this question, we first determined whether the central neurons that drive escape responses are active during EFPs. We expressed the genetically encoded fluorescent calcium reporter GCaMP in reticulospinal neurons in order to monitor firing. As previously described, vibrational stimuli activated a broad network of reticulospinal neurons, including the Mauthner cell. EFP stimuli also activated the Mauthner cell but failed to drive activity in other reticulospinal neurons. Ablation of the Mauthner cell completely abolished EFP responses, indicating that it is required for this behavior. Then, using genetic and pharmacological techniques, we isolated the Mauthner cell from synaptic input from other neurons, and found that it remained susceptible to activation by EFPs. We therefore proposed that EFPs bypass sensory cells and directly activate the Mauthner cell (2). This is likely because the Mauthner cell is a command neuron, which can drive an integrated behavioral response, and has the largest diameter axon in the nervous system of the fish. Consistent with this, EFPs selectively triggered responses when larvae were aligned such that the longitudinal axis of the Mauthner axon was parallel to the field. Understanding the mechanism by which EFPs trigger escape responses now enables us to probe Mauthner cell excitability non-invasively so that we can measure how different sensory stimuli and behavioral states affect escape responsiveness.
Figure 2. Larvae show ultra-rapid responses to electric field pulses
Image sequences comparing escape responses of zebrafish larva to an acoustic stimulus (left), and an electric field pulse (right). Time after the stimulus is indicated in milliseconds. Graph: Reaction time (latency to first movement in milliseconds) for acoustic (ac.) or electric (el.) responses. * p<0.05.
Tools for analyzing neuronal circuits that control behavior
We previously described a new method for restricting transgene expression to the nervous system, by incorporating a neuronal-restrictive silencing element. Using this method, we constructed a library of 240 Gal4 enhancer-trap transgenic fish with unique and restricted patterns of neuronal expression. The lines constitute an unparalleled resource for decoding the developmental genetics and neuroanatomical basis of behavior in zebrafish larvae (Figure 3). We mapped the integration site of the transgene in approximately 150 of these lines and found that, in about 15% of lines, the integration is in an exon or early intron of a gene, potentially disrupting protein expression. Indeed, in one such line, y241, larvae homozygous for the transgene insertion showed greatly reduced motility from the earliest stages of development. We found that, in this line, the transgene disrupted dual specificity phosphatase 27 (dusp27), which encodes a pseudophosphatase of previously uncharacterized biological function, and demonstrated that the near-paralysis in dusp27 mutants was attributable to a failure in the assembly of sarcomeric structures within muscle cells (3).
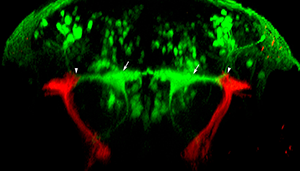
Figure 3. Gal4 enhancer trap lines for neuronal circuit analysis
Our library of Gal4 enhancer trap lines allow genetic targeting of reporter genes to specific neuronal cell types. One example is line y256, which labels stato-acoustic ganglion (SAG) neurons. In this transverse image through the hindbrain of a larva, y256 targets expression of a membrane-tagged red fluorescent protein to SAG neurons, allowing tracing of their projections into the brain. SAG neurons project dorsally and contact the bilateral pair of Mauthner cells (green, arrows), the command neurons for the startle response, at the distal tips of their lateral dendrites (arrowheads).
To complement these Gal4 lines that genetically target reporter genes to specific groups of neurons, we have also built a set of UAS (upstream activation sequence) reporter lines. To optimize expression from our UAS lines, we analyzed DNA sequence features of genes that are highly expressed in zebrafish. Based on this analysis, we wrote and validated new software that optimizes coding sequence for expression in zebrafish. We also tested non-coding sequence elements to identify introns and 3′ untranslated sequences that yield the strongest expression in zebrafish. We used this method to build strongly expressing UAS reporter lines to monitor and manipulate neurons, and we also generated transgenic UAS lines for two novel genetic tools. First, through codon optimization and introduction of amino acid substitutions, we developed a significantly more active version of the bacterial nitroreductase gene (epNTR) that is used for cellular ablation (2). We made a UAS:epNTR transgenic line that enables us to more rapidly and completely produce cell populations labeled in Gal4 lines, increasing our ability to detect behavioral deficits. To complement this loss-of-function approach, we also tested overexpression of the voltage-gated sodium channel SCN5 in order to sensitize neurons to synaptic input. After SCN5 expression in motor neurons, larvae showed larger angle escape responses but normal startle sensitivity, and conversely, after SCN5 expression in the Mauthner cell, larvae showed normal escape kinematics but a reduced response threshold (2). Together, the methods enable us to interrogate neuronal cell function in vivo and assess their contribution to behavior.
Publications
- Bergeron SA, Carrier N, Li GH, Ahn S, Burgess HA. Gsx1 expression defines neurons required for prepulse inhibition. Mol Psychiatry 2014;E-pub ahead of print.
- Tabor K, Bergeron SA, Horstick EJ, Jordan DC, Aho V, Porkka-Heiskanen T, Haspel G, Burgess HA. Direct activation of the Mauthner cell by electric field pulses drives ultrarapid escape responses. J Neurophysiol 2014;112:834-844.
- Fero K, Bergeron SA, Horstick E, Codore H, Li G, Ono F, Dowling J, Burgess HA. Impaired embryonic motility in dusp27 mutants reveals a developmental defect in myofibril structure. Dis Model Mech 2013;7:289-298.
- Fernandes AM, Fero K, Driever W, Burgess HA. Enlightening the brain: linking deep brain photoreception with behavior and physiology. BioEssays 2013;35:775-779.
- Ikeda H, Delargy A, Yokogawa T, Urban JM, Burgess HA, Ono F. Neuronal activity under ethanol-clamp in zebrafish revealed resistance to ethanol. PLoS One 2013;5:e63318.
Collaborators
- Kevin Briggman, PhD, Circuit Dynamics and Connectivity Unit, NINDS, Bethesda, MD
- Benjamin Feldman, PhD, Zebrafish Core Facility, NICHD, Bethesda, MD
- Michael J. Iadarola, PhD, Warren Grant Magnuson Clinical Center, NIH, Bethesda, MD
- Ralph Nelson, PhD, Basic Neurosciences Program, NINDS, Rockville, MD
- Mihaela Serpe, PhD, Program in Cellular Regulation and Metabolism, NICHD, Bethesda, MD
Contact
For more information, email haroldburgess@mail.nih.gov or visit ubn.nichd.nih.gov or neuroscience.nih.gov/Faculty/Profile/harold-burgess.aspx.