You are here: Home > Section on Cellular and Developmental Biology
Genes and Signals Regulating Mammalian Hematopoiesis

- Paul Love, MD, PhD, Head, Section on Cellular and Developmental Biology
- LiQi Li, MD, PhD, Research Fellow
- Amy Palin, PhD, Postdoctoral Fellow
- Claude Warzecha, PhD, Postdoctoral Fellow
- Zhenhu Li, PhD, Visiting Fellow
- Ekaterina Zvezdova, PhD, Visiting Fellow
- Dalal El-Khoury, BS, Technician
- Jan Lee, BS, Technician
Our research focuses on the development of the mammalian hematopoietic system. Of particular interest is the characterization of signal transduction molecules and pathways that regulate T cell maturation in the thymus. Current projects include the generation of transgenic and conditional deletion mutants to evaluate the importance of T cell antigen receptor signaling at specific stages of T cell development. We are also using microarray gene profiling to identify molecules that are important for thymocyte selection, a process that promotes the survival and further development of functional T cells and the death of auto-reactive T cells, thereby preventing autoimmunity. A newer project involves analyzing the function of Themis, a T cell–specific signaling protein recently identified by our laboratory. Another recently initiated area of investigation focuses on hematopoietic stem cells (HSCs), which give rise to all blood cell lineages. We have begun to characterize the genes that are important for the generation and maintenance of HSCs and for their differentiation into specific hematopoietic cell types. The studies revealed a critical function for one protein (Ldb1) in controlling the self-renewal/differentiation cell-fate decision in both HSCs and erythroblasts by acting as a key component of multi-subunit DNA–binding complexes. Global (ChIP-seq) screening for Ldb1-complex DNA–binding sites identified many targets for Ldb1–mediated regulation of transcription in hematopoietic cells, demonstrating an important role for Ldb1 in hematopoietic gene regulation. Current work on Ldb1 includes an examination of a potential role for this protein in regulating self-renewal of T cell progenitors in the thymus and in the genesis of T cell Acute Lymphoblastic Leukemia (T-ALL), one of the most common childhood malignancies.
T cell antigen receptor (TCR) signaling in thymocyte development
Click image to enlarge.
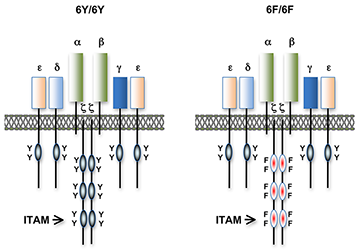
Figure 1. T cell antigen receptors expressed in 6Y/6Y and 6F/6F knock-in mice
Subunit composition of the T cell antigen receptors in 6Y/6Y and 6F/6F mice. 6Y/6Y mice express wild-type zeta chain dimers with functional ITAM signaling motifs that contain two tyrosine (Y) residues. 6F/6F mice express mutant zeta chain dimers in which the ITAM tyrosines have been changed to phenylalanine (F).
Much of our research focuses on the role of TCR signal transduction in thymocyte development. Signal transduction sequences, termed Immunoreceptor Tyrosine-based Activation Motifs or ITAMs, are contained within four distinct subunits of the multimeric TCR complex (CD3-zeta, CD3-gamma, CD3-delta, and CD3-epsilon). Di-tyrosine residues within ITAMs are phosphorylated upon TCR engagement; their function is to recruit signaling molecules, such as protein tyrosine kinases, to the TCR complex, thereby initiating the T cell–activation cascade. Though conserved, ITAM sequences are nonidentical, raising the possibility that the diverse developmental and functional responses controlled by the TCR may be partly regulated by distinct ITAMs. We previously generated CD3-zeta–deficient and CD3-epsilon–deficient mice by gene targeting. We genetically reconstituted the mice with transgenes encoding wild-type or signaling-deficient (ITAM–mutant) forms of CD3-zeta and CD3-epsilon and characterized the developmental and functional consequences of the alterations for TCR signaling. We found that TCR–ITAMs are functionally equivalent but act in concert to amplify TCR signals and that TCR signal amplification is critical for thymocyte selection, the process by which potentially useful immature T cells are instructed to survive and differentiate further (positive selection) and by which potentially auto-reactive cells that may cause auto-immune disease are deleted in the thymus (negative selection). Unexpectedly, we found that a complete complement of TCR–ITAMs is not required for mature T cell effector functions. One possible explanation is that multiple ITAM–mediated signal amplification is not required for mature T cell activation; another is that, in ITAM–mutant mice, T cells exhibit normal functional responsiveness because of compensatory mechanisms imposed during development. To resolve this question, we recently generated a TCR–zeta chain conditional knockin mouse in which T cell development and selection can occur without attenuation of TCR signaling (i.e., in the presence of wild-type 3 ITAM "6Y" zeta chain), but in which mature, post-selection T cells may be induced to express TCRs containing signaling-defective (0 ITAM "6F") zeta chains in lieu of wild-type zeta chains (Figure 1). Thus, mature T cell signaling should not be influenced by potential compensatory mechanisms that operate during T cell maturation, and T cells in these mice should be faithful indicators of the role of multiple TCR ITAMs in mediating specific, mature T cell responses. Experiments with the mice confirmed that the knockin zeta locus functions as predicted. We are currently using this model system to evaluate the role of ITAM multiplicity and ITAM–mediated signal amplification in T cell development, immune tolerance, and mature T cell function.
Identification and characterization of proteins important for TCR fine tuning and TCR signaling
We extended our analysis of TCR–signaling subunits to other molecules that participate in or influence the TCR–signaling response. The cell-surface protein CD5 negatively regulates TCR signaling and functions in thymocyte selection. Examination of CD5 expression during T cell development revealed that surface levels of CD5 are regulated by TCR signal intensity and by the affinity of the TCR for self peptide ligands in the thymus that mediate selection. To determine whether the ability to regulate CD5 expression is important for thymocyte selection, we generated transgenic mice that constitutively express high levels of CD5 throughout development. Overexpression of CD5 significantly impaired positive selection of some thymocytes (those that would normally express low levels of CD5) but not of others (those that would normally express high levels of CD5). The findings support a role for CD5 in modulating TCR signal transduction and thereby influencing the outcome of thymocyte selection. Current studies are centered on identifying the mechanism by which CD5 inhibits TCR signaling. The ability of individual thymocytes to regulate CD5 expression represents a mechanism for “fine tuning” the TCR signaling response during development so that the integrated signaling response can be adjusted to enable T cell functional competency without causing autoimmunity. Reasoning that, in addition to CD5, other molecules participate in TCR tuning, we initiated microarray-based screening for genes differentially expressed in developing T cells under conditions of high- or low-affinity TCR interactions. We identified several genes from this screen for further study. Given that these molecules regulate TCR signaling, they represent potential autoimmune-disease susceptibility markers and potential targets for treatment of patients with autoimmune disease.
Identification and characterization of Themis, a novel protein required for T cell development
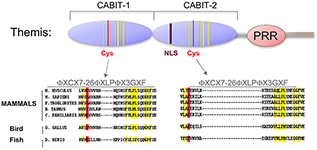
Using a subtractive cDNA library–screening approach, we recently identified Themis, a novel T cell–specific adapter protein (Figure 2). To investigate the function of Themis in T cell signaling and development, we generated Themis-knockdown cell lines, Themis-knockout mice, and Themis-transgenic mice. Analysis of the effects of modulating Themis expression revealed a critical role for the protein in late T cell development. Current data indicate that Themis functions in the signaling pathway downstream of the T cell receptor (TCR), perhaps by integrating or sustaining TCR signaling. Our ongoing studies are focusing on elucidating the mechanism through which Themis participates in T cell signaling and regulates T cell development.
Figure 2. Themis is highly conserved in vertebrates.
Themis contains two novel CABIT domains, each with a conserved cysteine (red) and conserved flanking residues (yellow), a nuclear localization signal (NLS), and a Proline-Rich Region (PRR).
Role of Ldb1 in hematopoiesis
Click image to enlarge.
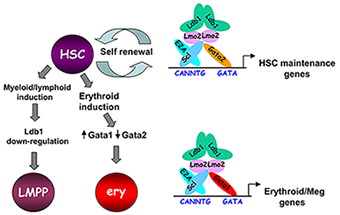
Figure 3. Model of Ldb1 function in the hematopoietic lineage
Ldb1 forms a multimeric DNA–binding complex in hematopoietic cells with the adapter Lmo2 and the transcription factors Scl and Gata1 or Gata2. In hematopoietic stem cells (HSCs), in which Gata2 is highly expressed, Ldb1-Lmo2-Scl-Gata2 complexes positively regulate expression of HSC maintenance genes. Differentiation of HSCs to the myeloid or lymphoid lineage (LMPP) is triggered by downregulation of Ldb1, whereas commitment to the erythroid lineage (ery) is triggered by induction of Gata1 and downregulation of Gata2, resulting in the formation of an Ldb1-Lmo2-Scl-Gata1 complex that positively regulates expression of erythroid-specific genes.
Lim domain binding protein-1 (Ldb1) is a ubiquitously expressed nuclear protein that contains a LIM–zinc finger protein interaction motif and a dimerization domain. In hematopoietic cells, Ldb1 functions by interacting with and/or recruiting specific partners (including the LIM–only protein Lmo2 and the transcription factors SCL/Tal1 and Gata1 or Gata2) to form multi-molecular transcription complexes (Figure 3). Within the hematopoietic lineage, expression of Ldb1 is highest in progenitor cells, which include hematopoietic stem cells (HSCs). Ldb1–null (Ldb1−/−) mice die between day 9 and 10 of gestation, preventing us from directly studying the impact of loss of Ldb1 on fetal or adult hematopoiesis. We investigated the role of Ldb1 in hematopoiesis by following the fate of Ldb1−/− embryonic stem (ES) cells in mouse blastocyst chimeras and by conditional, stage-specific deletion of Ldb1. Significantly, Ldb1−/− ES cells were capable of generating HSCs, which could give rise to both myeloid and lymphoid lineage cells; however, the number of Ldb1−/− HSCs gradually diminished at later stages of development. Following adoptive transfer of fetal liver hematopoietic progenitor cells, Ldb1−/− HSCs were rapidly lost, indicating a failure of self-renewal or survival. More recent data indicate that the loss of Ldb1−/− HSCs results from differentiation rather than cell death. Although expressed in ES cells, Ldb1 expression is not required for ES maintenance, indicating a selective requirement in adult stem cell populations. We performed a genome-wide screen for Ldb1–binding sites using ChIP-seq high-throughput sequencing. Analysis of ChIP-Seq data revealed that Ldb1 complexes bind at the promoter or regulatory sequences near a large number of genes known to be required for HSC maintenance. The data suggest that Ldb1 complexes function in a manner similar to Oct4/nanog/Sox2 in ES cells to regulate a core transcriptional network required for adult stem cell maintenance. Examination of the function of Ldb1 in lineages downstream of the HSC identified an essential function in the erythroid lineage but not in other myeloid cells or lymphoid cells. Interestingly, ChIP-Seq analysis of Ldb1 DNA–binding complexes demonstrated that, in HSCs, Ldb1 complexes contain Gata2 whereas, in erythroid progenitors, Ldb1 complexes contain Gata1 (which is highly expressed in the erythroid lineage). The results indicate that multimeric Ldb1 transcription complexes have distinct functions in the hematopoietic system depending on their subunit composition, with Gata2–containing complexes regulating expression of HSC–maintenance genes and Gata1 complexes regulating expression of erythroid-specific genes (Figure 3). Current studies are aimed at investigating how Ldb1 complexes regulate gene expression and the role of Ldb1 dimerization in mediating long-range promoter–enhancer interactions in hematopoietic cells. In addition, the lab is investigating a potential role for Ldb1 in regulating self-renewal of T cell progenitors in the thymus and in the genesis of T cell Acute Lymphoblastic Leukemia (T-ALL).
Additional Funding
- Dr. Amy Palin has applied for funding from the Helen Hay Whitney Foundation.
Publications
- Li L, Freudenberg J, Cui K, Dale R, Song SH, Dean A, Zhao K, Jothi R, Love PE. Ldb1-nucleated transcription complexes function as primary mediators of global erythroid gene activation. Blood 2013;121:4575-85.
- Zvezdova E, Lee J, El-Khoury D, Barr V, Akpan I, Samelson L, Love PE. In vivo functional mapping of the conserved protein domains within murine Themis1. Immunol Cell Biol 2014;92:721-728.
- Love PE, Warzecha C, Li L. Ldb1 complexes: the new master regulators of erythroid gene transcription. Trends Genet 2014;30:1-9.
- Smith S, Tripathi R, Goodings C, Cleveland S, Mathias E, Hardaway JA, Elliott N, Yi Y, Chen X, Downing J, Mullighan C, Swing DA, Tessarollo L, Li L, Love P, Jenkins NA, Copeland NG, Thompson MA, Du Y, Davé UP. LIM domain only-2 (LMO2) induces T-cell leukemia by two distinct pathways. PLoS One 2014;9:e85883.
- Vacchio MS, Wang L, Bouladoux N, Carpenter AC, Xiong Y, Williams LC, Wohlfert E, Song KD, Belkaid Y, Love PE, Bosselut R. A ThPOK-LRF transcriptional node maintains the integrity and effector potential of post-thymic CD4(+) T cells. Nat Immunol 2014;15:947-56.
Collaborators
- Avinash Bhandoola, PhD, University of Pennsylvania, Philadelphia, PA
- Remy Bosselut, PhD, Laboratory of Immune Cell Biology, NCI, Bethesda, MD
- Emery Bresnick, PhD, University of Wisconsin, Madison, WI
- Ann Dean, PhD, Laboratory of Cellular and Developmental Biology, NIDDK, Bethesda, MD
- Joshua Farber, MD, Laboratory of Clinical Investigation, NIAID, Bethesda, MD
- Sandra M. Hayes, MD, SUNY Upstate Medical University, Syracuse, NY
- Marc Jenkins, PhD, University of Minnesota, Minneapolis, MN
- Dorian McGavern, PhD, Viral Immunology and Intravital Imaging Section, NINDS, Bethesda, MD
- Alfred Singer, MD, Experimental Immunology Branch, NCI, Bethesda, MD
- Nan-ping Weng, PhD, Laboratory of Immunology, NIA, Baltimore, MD
- Keji Zhou, PhD, Immunology Center, NHLBI, Bethesda, MD
Contact
For more information, email lovep@mail.nih.gov.