You are here: Home > Section on Developmental Biology
Molecular Genetics of Embryogenesis in Xenopus and Zebrafish

- Igor B. Dawid, PhD, Head, Section on Developmental Biology
- Mi Ha Kim, PhD, Visiting Fellow
- Minho Won, PhD, Visiting Fellow
- Valeria Zarelli, PhD, Visiting Fellow
- Alison M. Heffer, PhD, Intramural Research Training Award Fellow
- Martha Rebbert, BS, Senior Technician
- John Gonzales, MA, MS, Technician
- Nathaniel A. Parker, BA, Postbaccalaureate Student
The laboratory uses the frog Xenopus laevis and the zebrafish Danio rerio as experimental systems to study molecular-genetic mechanisms of early vertebrate development. Recently, we focused on mechanisms of caudal body development, neural crest specification, pancreas development, and targeted gene disruption.
Modulation of Tcf3 repressor complex composition regulates cdx4 expression and caudal body formation in zebrafish.
Click image to enlarge.
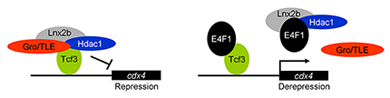
Figure 1. E4f1 enhances cdx4 expression by destabilizing the repressor complex between Tcf3 and the co-repressors HDAC1 and Groucho/TLE.
In this model, the left drawing shows the repressor complex. Tcf3 occupies cis-regulatory sites in the cdx4 gene while binding to co-repressors HDAC1 and TLE; the complex is stabilized by Lnx-2b. The right drawing shows de-repression by E4f1. E4f1 dissociates the complex through its ability to bind to Tcf3, HDAC1, and Lnx-2b. Tcf3 remains bound to the DNA but no longer exerts repressive activity.
Development of the caudal body region depends on several signaling pathways, among which the Wnt pathway plays a prominent role. The Wnt signal is ultimately mediated by the Cdx family of homeodomain proteins, with Cdx4 being the most important member in zebrafish. In all vertebrates, including humans, Cdx factors are critical for the activation of posterior Hox genes and, among other processes, for hematopoiesis. We studied the modulation of cdx4 gene expression by E4f1, a factor known as a repressor of cyclin A2 transcription. We found that E4f1 cooperates with Wnt signaling in promoting the expression of the cdx4 gene and is consequently required for caudal development (1). We studied the molecular mechanism of this effect, concluding that E4f1 modulates the activity of Tcf3. The canonical Wnt pathway exerts its effects by controlling the activity of transcription factors of the Lef/Tcf family. In the absence of Wnt signaling, Tcf3 acts as a repressor of Wnt target genes in the early zebrafish embryo; Wnt relieves this repression. We found that E4f1 acts by modulating stability of the Tcf3 repressor complex by dissociating co-repressors such as HDAC1 and Groucho/TLE from Tcf3 (Figure 1). We suggest that E4f1 acts as a modulator of cdx4 expression to ensure the robust nature of the gradient of Cdx4 activity that is necessary for normal formation of the caudal body region and hematopoiesis (1).
The BTB domain protein Kctd15 restricts the neural crest domain in zebrafish and Xenopus embryos.
Click image to enlarge.
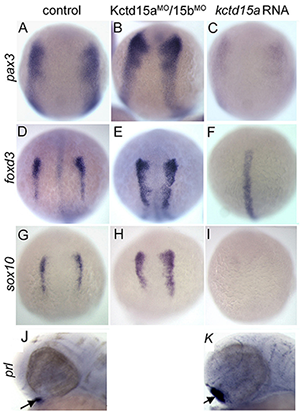
Figure 2. Kctd15 inhibits neural crest specification in zebrafish.
A-I: In situ hybridization of early somite embryos show that markers of neural plate border (pax3) and neural crest (foxd3, sox10) are inhibited by Kctd15 overexpression and enhanced by its knock-down. J, K: In contrast, Kctd15 overexpression enhances the formation of anterior placode derivatives such as the pituitary gland, marked by prl. Figure adapted from Dutta S, Dawid IB, Development 2010;137:3013.
Our laboratory has a long-standing interest in the formation of the neural crest (NC), a group of cells with stem cell properties that arise at the dorsal neural tube and migrate to many locations in embryos to give rise to a large number of varied differentiated derivatives. A recent focus has been the role of the BTB domain–containing protein Kctd15 in regulating the NC domain in the embryo. Kctd15 is first expressed at the neural plate border, where the NC and the pre-placodal domains are specified. Overexpression of Kctd15 strongly inhibits NC specification, as seen by the suppression of several genes that mark the NC domain in whole embryos (Figure 2). At the same time, knock-down of Kcdt15 by antisense morpholinos enhances the expression of these marker genes. Both the pre-placodal and NC domains form at the neural plate border, and mechanisms should exist that prevent one domain from invading the territory of the other. Kctd15 could be involved in such a restricting mechanism. This reasoning suggests that Kctd15 overexpression might enhance placode development while inhibiting the NC. We found this to be true for anterior placodes, as illustrated for pituitary development by the enhanced expression of the marker gene prl (Figure 2: J,K). Thus, we propose that Kctd15 is involved in delineating the separate pre-placodal and NC domains at the neural plate border during embryogenesis. In pursuing the molecular mechanism of Kctd15 action, we find that this factor regulates the activity of transcription factor AP-2.
Med12 is required for hindbrain organization.
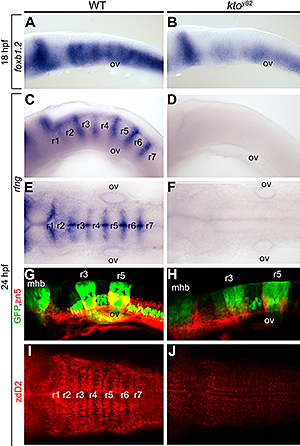
We characterized a mutation named kohtalo that affects the Mediator component Med12. Previously we showed that this mutation affects several tissues in the embryo. We found (2) that the Med12 mutant shows disturbed organization of the hindbrain (Figure 3). We conclude that hindbrain segmentation per se is retained in the kohtalo mutant zebrafish, but the properties of the segments are disturbed.
Figure 3. Specific loss of hindbrain boundaries in ktoy82 mutants.
Lateral (A–D, G, H) and dorsal views (E, F, I, J) of wt (A, C, E, G, I) and ktoy82 mutant embryos (B, D, F, H, J) at 18 hpf (A, B) and 24 hpf (C–J). A–F: In situ hybridization. A, B: Expression of the earliest hindbrain boundary marker foxb1.2. C–F: Complete loss of rfng expression in hindbrain boundaries. G, H: Confocal images in transgenic zebrafish expressing GFP (green) in rhombomeres 3 and 5, immunostained with zn5 to visualize hindbrain boundary neurons (red). I, J: Staining with zebrafish delta D antibody, staining paraboundary regions. While rhombomere segmentation per se is maintained, boundary and paraboundary staining in mutant embryos is reduced or lost.
Formation of the yolk syncytial layer in zebrafish
The yolk syncytial layer (YSL) is an extra-embryonic tissue that is important in zebrafish development. The formation of this tissue involves cell fusion. Regulation of the arrangements of syncytial nuclei and the function of microtubules are involved in YSL formation. The processes have significant roles in multiple contexts. In a collaborative study, we elucidated the function of a molecule, Slc3a2, that is required for normal YSL formation (3). The work has implications for the pathways that regulate microtubule organization and cell fusion beyond the specific context of YSL formation.
The role of the homeodomain factor Hhex in pancreas development
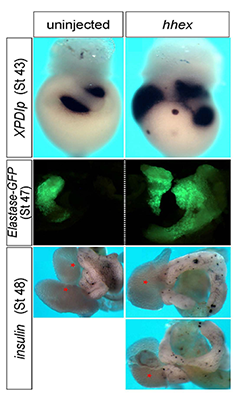
One of the factors that emerged from our screening efforts is vvp1. In a collaborative effort, we found that the vvp1 gene is an excellent marker for a population of early ventral pancreatic precursors (4). Using this novel marker gene, we studied pancreas development in Xenopus embryos, focusing on the regulatory function of the homeobox gene hhex. In tail bud embryos, vpp1 expression can be used to specifically label two ventral pancreatic buds, whereas hhex expression is primarily seen in the liver diverticulum. Ectopic over-expression of a carefully titrated level of hhex mRNA led to a substantial increase in the domain of vpp1-positive cells, which later led to increased development of the ventral pancreas (Figure 4). The giant ventral pancreata may arise by a re-specification of intestinal precursor cells to ventral pancreatic precursor cells. In using antisense morpholino oligonucleotides to study loss-of-function phenotypes, the knockdown of hhex led to a reduction of the expression of the vpp1 gene and the specific interference with the development of the ventral pancreas. On the basis of these observations, we suggest that hhex plays a critical role in the formation of a vpp1-positive cell population in the endoderm of the gastrula embryo that is essential for the subsequent development of the ventral pancreas.
Figure 4. hhex overexpression increases pancreas differentiation.
In situ hybridization with Pdlp, and inspection of elastase-GFP transgenic frogs, showed enlarged exocrine tissue. Insulin staining visualized scattered endocrine cells in a similar pattern as in the control but in a much expanded tissue. As seen in the right-bottom two images, hhex–overexpressing embryos often formed annular pancreata.
Targeted gene disruption in Xenopus using TALENs
The frog Xenopus has been a valuable model system in developmental biology for a long time. I cannot fail to mention that I write this paragraph the day after John Gurdon was awarded the Nobel Prize for work in Xenopus. However, in this model as in the zebrafish, the lack of techniques for targeted gene disruption has been a major limitation to progress. Recent developments are about to change this situation. Transcription activator-like effectors (TALEs), discovered in plant pathogenic bacteria, are DNA–binding proteins with a simple code that has been deciphered, allowing the generation of DNA–binding modules for any target sequence. TALE-nuclease hybrid proteins (TALENs) are able to cut the chromosome within the nucleus at the targeted site, leading to repair that often introduces deletions or insertions, disrupting the gene. In a collaborative effort, we applied and adapted the TALEN technology to Xenopus, and showed that it functions at high efficiency in this system (5). We hope that this work will encourage other laboratories working with Xenopus to apply this highly promising method.
Publications
- Ro H, Dawid IB. Modulation of Tcf3 repressor complex composition regulates cdx4 expression in zebrafish. EMBO J 2011;30:2894-2907.
- Hong SK, Dawid IB. The transcriptional mediator component med12 is required for hindbrain boundary formation. PLoS One 2011;6:e19076.
- Takesono A, Moger J, Faroq S, Cartwright E, Dawid IB, Wilson SW, Kudoh T. Slc3a2 controls yolk syncytial layer (YSL) formation by regulating microtubule networks in the zebrafish embryo. Proc Natl Acad Sci USA 2012;109:3371-3376.
- Zhao H, Han D, Dawid IB, Pieler T, Chen Y. Homeoprotein hhex-induced conversion of intestinal to ventral pancreatic precursors results in the formation of giant pancreata in Xenopus embryos. Proc Natl Acad Sci USA 2012;109:8594-8599.
- LeiY, Guo X, Liu Y, Cao Y, Deng Y, Chen X, Ki H, Cheng C, Dawid IB, Chen Y, Zhao H. Efficient targeted gene disruption in Xenopus embryos using engineered transcription activator-like effector nucleases (TALENs). Proc Natl Acad Sci USA 2012;109:E-pub alead of print.
Collaborators
- Tetsuhiro Kudoh, PhD, Exeter University, Exeter, UK
- Hui Zhao, PhD, The Chinese University, Hong Kong, China
Contact
For more information, email dawidi@mail.nih.gov or visit sdb.nichd.nih.gov.