You are here: Home > Section on Developmental Biology
Molecular Genetics of Embryogenesis in Xenopus and Zebrafish

- Igor B. Dawid, PhD, Head, Section on Developmental Biology
- Reiko Toyama, PhD, Staff Scientist
- Hyunju Ro, PhD, Research Fellow
- Mi Ha Kim, PhD, Visiting Fellow
- Minho Won, PhD, Visiting Fellow
- Valeria Zarelli, PhD, Visiting Fellow
- Martha Rebbert, BS, Senior Technician
- John Gonzales, MA, MS, Technician
The laboratory uses the frog Xenopus laevis and the zebrafish Danio rerio as experimental systems to study molecular-genetic mechanisms of early vertebrate development. Recently, we focused on mechanisms of gastrulation, caudal body development, and neural crest specification.
The E3 ubiquitin ligase Lnx-2b regulates organizer size in the zebrafish embryo.
Click image to enlarge.
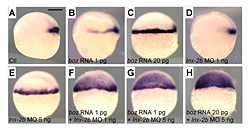
Figure 1. Levels of Boz and Lnx-2b control the domain of goosecoid (gsc) expression.
A. Control embryo; gsc is expressed in the organizer. B. Injection of 1 pg of boz mRNA causes lateral expansion of gsc. C. Injection of 20 pg of boz mRNA causes expansion to the entire margin. D. Injection of 1 ng of lnx-2b morpholino antisense oligonucleotide gives slight lateral expansion of gsc. E. Injection of 5 ng MO results in full marginal expansion. F–H. boz mRNA and lnx-2b MO cooperate in a dose-dependent way to expand the gsc domain into the presumptive ectoderm. Lateral views, dorsal is right. Scale bar, 200 mm.
Click image to enlarge.
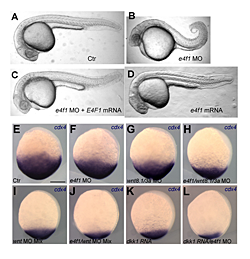
Figure 2. E4f1 cooperates with Wnt signaling in supporting caudal development and cdx4 expression.
A–D. Morpholino (MO)–mediated knock-down of E4f1 leads to shortened tails (B). The effect can be rescued by mRNA injection (C). E-L. Knock-down of E4f1 and of Wnt signaling by MO or the Wnt antagonist Dkk cooperate in progressively reducing cdx4 expression in bud-stage zebrafish embryos.
In the vertebrate embryo, the organizer anchors the primary embryonic axis, and balance between the dorsal (organizer) and ventral domains is fundamental to body patterning. We have studied a mechanism that regulates the size of the organizer domain by modulating the stability of a key molecule involved in organizer formation. This regulatory pathway is mediated by a member of the Ligand of Numb protein-X (Lnx) family, a RING finger, and four PDZ domains containing the E3 ubiquitin ligase. Earlier, we described how Lnx-2b acts as a critical regulator of dorso-ventral axis formation in the zebrafish embryo. We identified Bozozok (Boz; also called Dharma or Nieuwkoid) as a binding partner and substrate of Lnx-2b. Boz is a homeodomain-containing transcriptional repressor induced by canonical Wnt signaling that is critical for the formation of the dorsal organizer. Lnx-2b induced lysine-48–linked polyubiquitylation of Boz, leading to its proteasomal degradation. More recently, we studied the regulation of the organizer gene goosecoid by the interaction of Lnx-2b and Bozozok (1) and showed that the balance between Lnx-2b and Boz delimits the goosecoid domain in the gastrula embryo (Figure 1). The studies introduce a ubiquitin ligase, Lnx-2b, as a balancing modulator of axial patterning in the zebrafish embryo and emphasize the role of post-translational regulation of protein stability in early embryogenesis.
Modulation of Tcf3 repressor complex composition regulates cdx4 expression and caudal body formation in zebrafish.
Click image to enlarge.
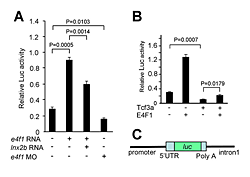
Figure 3. E4f1 stimulates Cdx4 reporter activity.
A. Basal activity of a Cdx4 reporter construct, shown in C, is stimulated by E4f1 in 293T cells while Lnx-2b inhibits the effect. B. Tcf3 inhibits Cdx4 reporter activity, and E4f1 leads to de-repression.
Click image to enlarge.
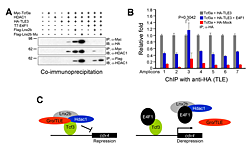
Figure 4. E4f1 destabilizes the repressor complex between Tcf3 and the co-repressors HDAC1 and Groucho/TLE.
A. In 293T cells, interaction between Tcf3 and HDAC1 or TLE can be seen in co-immunoprecipitation experiments; these complexes are stabilized by Lnx-2b but dissociated by E4f1. B. ChIP experiments show that TLE binds to Tcf3 sites in the regulatory region of the cdx4 gene but is removed from these sites by the addition of E4f1. C. A model for the mechanism of E4f1 de-repression of the cdx4 gene. Left drawing, repressor complex. Tcf3 occupies cis-regulatory sites in the cdx4 gene while binding to co-repressors HDAC1 and TLE; the complex is stabilized by Lnx-2b. Right drawing, de-repression by E4f1. E4f1 dissociates the complex through its ability to bind to Tcf3, HDAC1, and Lnx-2b. Tcf3 remains bound to the DNA but no longer exerts repressive activity.
Development of the caudal body region depends on several signaling pathways, among which the Wnt pathway plays a prominent role. The Wnt signal ultimately is mediated by the Cdx family of homeodomain proteins, with Cdx4 the most important member in zebrafish. In all vertebrates, including humans, Cdx factors are critical for the activation of posterior Hox genes and, among other processes, for hematopoiesis. We studied the modulation of cdx4 gene expression by Lnx-2b, the ubiquitin ligase discussed above, and E4f1, a factor known as a repressor of cyclin A2 transcription. We found (2) that E4f1 cooperates with Wnt signaling in promoting the expression of the cdx4 gene, and consequently is required for caudal development (Figure 2). We studied the effect further by using a reporter construct in which the cdx4 promoter controls luciferase expression. E4f1 stimulates cdx4 expression whereas Lnx-2b, the ubiquitin ligase discussed above, counteracts this stimulation (Figure 3). The canonical Wnt pathway exerts its effects by controlling the activity of transcription factors of the Lef/Tcf family. It is known that Tcf3 acts as a repressor of Wnt target genes in the early zebrafish embryo. We found that Tcf3 repressed the cdx4 reporter and that E4f1 could alleviate such respression, i.e., could de-repress the cdx4 (Figure 3). The mechanism of E4f1 modulation of cdx4 expression does not involve direct binding of E4f1 to the DNA, but rather modulation of the repressor complex by protein-protein interactions. Tcf3 associates with co-repressors such as HDAC1 and Groucho/TLE in repressing cdx4. We found that E4f1 can dissociate the repressor complex without removing Tcf3 from its cognate sites on the DNA whereas Lnx-2b, acting as a scaffold protein irrespective of its enzymatic activity, stabilizes the complex (Figure 4). Through this activity, E4f1 has the capacity to de-repress the cdx4 gene. We suggest that E4f1 acts as a modulator of cdx4 expression to ensure the robust nature of the gradient of Cdx4 activity necessary for normal formation of the caudal body region and hematopoiesis (2).
The BTB domain protein Kctd15 restricts the neural crest domain in zebrafish and Xenopus embryos.
Click image to enlarge.
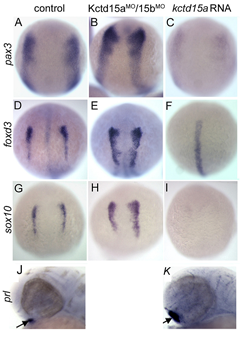
Figure 5. Kctd15 inhibits neural crest specification in zebrafish.
In situ hybridization of early somite embryos shows that markers of neural plate border (pax3) and neural crest (foxd3, sox10) are inhibited by Kctd15 overexpression and enhanced by its knock-down (A–I). In contrast, Kctd15 over-expression enhances the formation of anterior placode derivatives such as the pituitary gland, marked by prl (J,K).
Our laboratory has a long-standing interest in the formation of the neural crest (NC), a group of cells with stem cell properties; the cells arise at the dorsal neural tube and migrate to many locations in the embryo to give rise to a large number of varied, differentiated derivatives. Recently, we have been focussing on the role of the BTB domain–containing protein Kctd15 in regulating the NC domain in the embryo (3). Kctd15 is first expressed at the neural plate border, where the NC and the pre-placodal domains are specified. Over-expression of Kctd15 strongly inhibits NC specification, as seen by the suppression of several genes that mark the NC domain in whole embryos (Figure 5). At the same time, knock-down of Kcdt15 by antisense morpholinos (MOs) enhances the expression of these marker genes (Figure 5). Both the NC and pre-placodal domains form at the neural plate border, and mechanisms should exist that prevent one domain from invading the territory of the other. Kctd15 could be involved in such a restricting mechanism. This reasoning suggests that Kctd15 over-expression might enhance placode development while inhibiting the NC. We found this to hold for anterior placodes, as illustrated for pituitary development by the enhanced expression of the marker gene prolactin (Figure 5, J and K). Thus, we propose that Kctd15 is involved in delineating the separate NC and pre-placodal domains at the neural plate border during embryogenesis (3).
Publications
- Ro H, Dawid IB. Lnx-2b restricts gsc expression to the dorsal mesoderm by limiting Nodal and Bozozok activity. Biochem Biophys Res Commun 2010;402:626-630.
- Ro H, Dawid IB. Modulation of Tcf3 repressor complex composition regulates cdx4 expression in zebrafish. EMBO J 2011;30:2894-2907.
- Dutta S, Dawid IB. Kctd15 inhibits neural crest formation by attenuating Wnt/β-catenin signaling output. Development 2010;137:3013-3018.
- Aamar E, Dawid IB. Sox17 and chordin are required for formation of Kupffer's vesicle and left-right asymmetry determination in zebrafish. Dev Dynamics 2010;239:2980-2988.
- Hong SK, Dawid IB. The transcriptional mediator component med12 is required for hindbrain boundary formation. PLoS One 2011;6:e19076.
Collaborators
- Neil A. Hukriede, PhD, University of Pittsburgh, Pittsburgh, PA
- Tetsuhiro Kudoh, PhD, Exeter University, Exeter, UK
- Kosuke Tanegashima, PhD, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan
- Michael Tsang, PhD, University of Pittsburgh, Pittsburgh, PA
- Hui Zhao, PhD, The Chinese University, Hong Kong, China
Contact
For more information, email dawidi@mail.nih.gov or visit sdb.nichd.nih.gov.

