You are here: Home > Section on Vertebrate Organogenesis
Organogenesis of the Zebrafish Vasculature

- Brant M. Weinstein, PhD, Head, Section on Vertebrate Organogenesis
- Matthew Butler, PhD, Leducq Postdoctoral Fellow
- Aniket Gore, PhD, Leducq Postdoctoral Fellow
- Young Cha, PhD, Postdoctoral Fellow
- Misato Fujita, PhD, Postdoctoral Fellow
- Shlomo Krispin, PhD, Postdoctoral Fellow
- Katherine Monzo, PhD, Postdoctoral Fellow
- Timothy Mulligan, PhD, Postdoctoral Fellow
- Weijun Pan, PhD, Postdoctoral Fellow
- Matthew Swift, PhD, Postdoctoral Fellow
- Jianxin Yu, PhD, Postdoctoral Fellow
- Van Pham, BS, Scientific Technician
The overall objective of this project is to understand how the elaborate networks of blood and lymphatic vessels arise during vertebrate embryogenesis. Blood vessels supply every tissue and organ with oxygen, nutrients, and cellular and humoral factors. Lymphatic vessels drain fluids and macromolecules from the interstitial spaces of tissues, returning them to the blood circulation, and play an important role in immune responses. Understanding the formation of blood and lymphatic vessels is engendering intense clinical interest because of the roles that both types of vessels play in cancer and ischemia. The zebrafish, a small tropical freshwater fish, possesses a unique combination of features that make it particularly suitable for studying vessel formation; the fish is a genetically tractable vertebrate with a physically accessible, optically clear embryo. These features are highly advantageous for studying vascular development, permitting observation of every vessel in the living animal and simple, rapid screening for even subtle vascular-specific mutants.
Major aims of the laboratory include developing new tools for studying vascular development in zebrafish, experimental analysis of vascular morphogenesis, vascular patterning, and lymphatic development, and forward-genetic analysis of vascular development.
Developing tools for experimental analysis of vascular development in the zebrafish
Click image to enlarge.
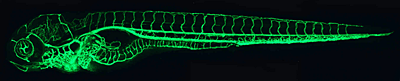
Figure 1. The zebrafish vascular system
Confocal microangiogram of the vascular system of a 4-1/2-day-old zebrafish larva labeled by injection of fluorescent microspheres. The transparency of zebrafish larvae makes it possible to use high-resolution optical imaging methods to visualize the entire vasculature in exquisite detail.
The development of new tools to facilitate vascular studies in the zebrafish is an important ongoing aim of this project. Previously, we (i) established a microangiographic method for imaging patent blood vessels in the zebrafish and used this method to compile a comprehensive staged atlas of the vascular anatomy of the developing fish (http://zfish.nichd.nih.gov/zfatlas/Intro Page/intro1.html); (ii) generated a variety of transgenic zebrafish lines expressing various fluorescent proteins within vascular or lymphatic endothelial cells, making it possible for us to visualize vessel formation in intact, living embryos; and (iii) developed methodologies for long-term multiphoton confocal time-lapse imaging of vascular development in transgenic fish. Recent technical advances have greatly facilitated the generation of new transgenic lines in the fish, and we are currently developing many new lines useful for in vivo vascular imaging as well as for in vivo endothelial-specific functional manipulation of signaling pathways involved in vascular specification, patterning, and morphogenesis.
Genetic analysis of vascular development
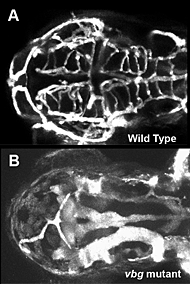
Figure 2. Cranial vessel malformation in violet beauregarde (vbg) mutant zebrafish
Dorsal view of cranial vessels in 2-1/2-day-old wild-type (A) and vbg mutant (B) zebrafish larvae, showing reduced number of highly enlarged and malformed vessels. Vbg mutants are defective in acvrl1, a gene implicated in hereditary hemorrhagic telangiectasia (HHT), a human inherited vascular malformation disorder.
We use forward-genetic approaches to identify and characterize new zebrafish mutants that affect the formation of the developing vasculature. Using transgenic zebrafish expressing green fluorescent protein (GPF) in blood vessels (Figure 1), we are carrying out ongoing large-scale genetic screens for mutants induced by N-ethylN-nitrosourea (ENU). To date, we have screened well over 2,000 genomes and identified over 100 new vascular mutants with phenotypes that include loss of most vessels or subsets of vessels, increased sprouting/branching, and vessel mispatterning. Most recently, we carried out a new genetic screen to identify hemorrhagic stroke–susceptibility genes. A bulked segregant mapping pipeline is in place to determine rapidly the rough position of newly identified mutants on the zebrafish genetic map. Fine mapping and molecular cloning are in progress for many mutants. We previously positionally cloned the defective genes from several vascular-specific mutants, including violet beauregarde (defective inAlk1/acvrl1; Figure 2), plcg1 (defective in phospholipase C-gamma 1), kurzschluss (defective in a novel chaperonin), beamter (defective in trunk somite and vascular patterning), and etsrp (defective in an ETS1−related transcription factor). We are currently focusing on several mutants affecting VEGF2−dependent and VEGF-independent vascular signaling pathways. Our ongoing mutant screens and positional cloning projects continue to yield a rich harvest of novel vascular mutants and genes, bringing to light new pathways regulating the formation of the developing vertebrate vasculature.
1E26 transformation-specific
2Vascular endothelial growth factor
Analysis of vascular morphogenesis and integrity
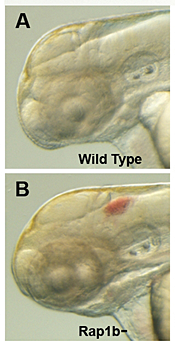
Figure 3. Intracranial hemorrhage (ICH) in the developing zebrafish
The clarity of zebrafish larvae also makes it straightforward to screen for animals with intracranial hemorrhage, as is evident in comparing lateral views of a 2-day-old wild-type larva (A) with a hemorrhage-prone larva deficient in rap1b (B).
Proper morphogenesis of vascular tubes and the maintenance of their integrity is critically important to human health. Malformation or rupture of vessels is the basis for stroke, the third leading cause of death and the most common cause of disability in developed nations. Intracerebral hemorrhage (ICH) accounts for 10 percent of stroke and is a particularly severe form of the disease, with disproportionately high rates of death and long-term disability. Therapeutic tools are still very limited, and prevention remains the most important way to reduce morbidity and mortality. In previous studies, we used high-resolution time-lapse two-photon imaging to examine vessel morphogenesis in living zebrafish, showing that the formation and intra- and intercellular fusion of endothelial vacuoles drives vascular lumen formation in vivo. We are currently examining the formation and maintenance of vascular endothelial cell-cell junctions, which are particularly important in stroke. To begin dissecting the molecular regulatory mechanisms controlling endothelial junction formation, we examined a variety of genes required for vascular morphogenesis and vascular integrity, including the ccm, pak2a, and rap1b genes (see Figure 3 for the effects of rap1b disruption). We are also developing transgenic lines that permit us to visualize the dynamics of endothelial cell-cell junctions and intracellular cytoskeletal structures in order to examine their role in the cellular rearrangements that occur during vascular sprouting and growth and vascular tube formation. As noted above, we have also carried out a genetic screen for genes that increase susceptibility to ICH. This screen identified several dozen new mutants that raise the propensity for ICH. Positional cloning of the affected genes will identify novel genetic modifiers of the onset and severity of hemorrhagic stroke, potentially leading to the development of new therapeutic targets for the treatment and prevention of human ICH.
Analysis of vascular patterning
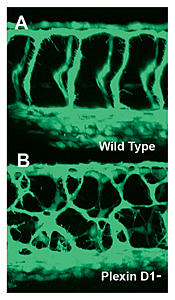
Figure 4. Mispatterned trunk vessels in larvae lacking the vascular semaphorin receptor plexin D1
Confocal imaging of trunk vessels in a 2-1/2-day-old wild-type (A) and a plexin D1–deficient (B) larva, showing loss of proper patterning of the trunk vessels caused by inability to receive semaphorin repulsive guidance signals.
We used multiphoton time-lapse imaging to characterize patterns of vessel assembly throughout the developing zebrafish. Ongoing studies in the laboratory aim to understand how these patterns arise and what cues guide vascular network assembly during development. We previously demonstrated that known neuronal guidance factors play an important, previously unknown role in vascular guidance and vascular patterning, showing that semaphorin signaling is an essential determinant of trunk blood vessel patterning (Figure 4). Current studies are further elucidating the role of additional factors that guide the patterning of developing blood and lymphatic vascular networks in vivo, both in the trunk and in vascular beds in the eye, aortic arches, hindbrain, and other anatomical locales.
Analysis of lymphatic development
The lymphatic system has become the subject of great interest in recent years because of the recognition of its important role in normal and pathological processes, but progress in understanding the origins and early development of the system has been hampered by difficulties in observing lymphatic cells in vivo and performing defined genetic and experimental manipulation of the lymphatic system in currently available model organisms. We recently demonstrated that the zebrafish possesses a lymphatic system that shares many of the morphological, molecular, and functional characteristics of the lymphatic vessels found in other vertebrates, providing a powerful new model for imaging and studying lymphatic development. As we continue to examine the origins and assembly of the lymphatic system of the zebrafish, we are developing new transgenic tools for imaging the development of the lymphatic system and for forward-genetic screening for lymphatic mutants. Our genetic analysis has already identified several novel genes involved in lymphatic development and patterning. We are also studying the roles of a number of different genes required for specification, assembly, or patterning of the lymphatic endothelium. Our ongoing studies will provide new insights into the molecular regulation of lymphatic development.
Additional Funding
- Fondation Leducq (2007), Transatlantic Network of Excellence for the Identification of Novel Genetic Targets in Hemorrhagic Stroke, to American Coordinator Dr. Brant Weinstein (ongoing)
- JSPS Fellowship (2008), to Dr. Misato Fujita
Publications
- Castranova D, Lawton A, Lawrence C, Baumann D, Best J, Doherty A, Ramos J, Wang C, Wilson C, Malley J, Weinstein BM. The effect of stocking densities on reproductive performance in laboratory zebrafish (Danio rerio). Zebrafish 2011;8:141-146.
- Fujita M, Cha YR, Roman BL, Weinstein BM. Assembly and patterning of the vascular network of the vertebrate hindbrain. Development 2010;138:1705-1715.
- Gore AV, Swift M, Cha YR, Lo BD, McKinney MC, Li W, Castranova D, Davis A, Mukouyama Y, Weinstein BM. Vegfc/Vegfr3-dependent angiogenesis is regulated by canonical Rspo1/Wnt signaling. Development 2011;138:4875-4886.
- Lim AH, Suli A, Yaniv K, Weinstein BM, Li DY, Chien C-B. Motoneurons are essential for vascular pathfinding. Development 2011;138:3847-3857.
- Miskinyte S, Butler M, Hervé H, Sarret C, Nicolino M, Evans JD, Bergametti F, Arnould M, Pham VN, Gore AV, Spengos K, Gazal S, Woimant F, Steinberg GK, Weinstein BM, Tournier-Lasserve E. Loss of the BRCC3 deubiquitinating enzyme leads to abnormal angiogenesis and is associated with moyamoya angiopathy. Am J Hum Genet 2011;88:718-728.
Collaborators
- Chi-Bin Chien, PhD, University of Utah, Salt Lake City, UT
- George Davis, PhD, University of Missouri-Columbia, Columbia, MO
- Elisabetta Dejana, PhD, The FIRC Institute of Molecular Oncology Foundation, Milan, Italy
- Louis Dye, BS, Microscopy and Imaging Core, NICHD, Bethesda, MD
- Steven Farber, PhD, Carnegie Institute, Baltimore, MD
- Luisa Iruela-Arispe, PhD, University of California Los Angeles, Los Angeles, CA
- Sumio Isogai, PhD, Iwate Medical University, Morioka, Japan
- Pudur Jagadeeswaran, PhD, University of North Texas, Dallas-Fort Worth, TX
- Nathan Lawson, PhD, University of Massachusetts, Worcester, MA
- Dean Li, MD, University of Utah, Salt Lake City, UT
- Paul Liu, MD, PhD, Genetics and Molecular Biology Branch, NHGRI, Bethesda, MD
- James Malley, PhD, Center for Information Technology, NIH, Bethesda, MD
- Philip Murphy, MD, Laboratory of Molecular Immunology, NIAID, Bethesda, MD
- Yohsuke Mukuoyama, PhD, Genetics & Developmental Biology Center, NHLBI, Bethesda, MD
- Beth Roman, PhD, University of Pittsburgh, Pittsburgh, PA
- Gregory Shelness, MD, Wake Forest University, Winston-Salem, NC
- Arndt Siekmann, PhD, Max Planck Institute for Molecular Biomedicine, Münster, Germany
- Elizabeth Tournier-Lasserve, PhD, Inserm (Lariboisiere), Paris, France
Contact
For more information, email weinsteb@mail.nih.gov or visit uvo.nichd.nih.gov.