Quantitative Imaging and Tissue Sciences

- Peter J. Basser, PhD, Head, Section on Quantitative Imaging and Tissue Sciences
- Ferenc Horkay, PhD, Staff Scientist
- Sinisa Pajevic, PhD, Staff Scientist (CIT)
- Magdoom Kulam, PhD, Postdoctoral Visiting Fellow
- Matan Mussel, PhD, Postdoctoral Visiting Fellow
- Velencia Witherspoon, PhD, Postdoctoral Intramural Research Training Award (IRTA) Fellow
- Nathan Hu Williamson, PhD, PRAT Postdoctoral Intramural Research Training Award Fellow (NIGMS)
- Teddy Cai, BS, Predoctoral Intramural Research Training Award Fellow, NIH OxCam Program
- Alexandru Avram, PhD, Collaborating Scientist funded via the Henry Jackson Foundation and the Center for Neuroscience and Regenerative Medicine
- Dan Benjamini, PhD, Collaborating Scientist funded via the Henry Jackson Foundation and the Center for Neuroscience and Regenerative Medicine
- Michal Komlosh, PhD, Collaborating Scientist funded via the Henry Jackson Foundation and the Center for Neuroscience and Regenerative Medicine
- Kadharbatcha Saleem, PhD, Collaborating Scientist funded via the Henry Jackson Foundation and the Center for Neuroscience and Regenerative Medicine
- Alexandros Chremos, PhD, Contract Scientist
- Rea Ravin, PhD, Contract Scientist
In our tissue sciences research, we strive to understand fundamental relationships between function and structure in living tissues. Specifically, we are interested in how tissue microstructure, hierarchical organization, composition, and material properties affect their biological function or dysfunction. We investigate biological and physical model systems at various length and time scales, performing biophysical measurements in tandem with developing physical/mathematical models to explain their functional properties and behavior. Experimentally, we use water to probe tissue structure and function from nanometers to centimeters and from microseconds to lifetimes. We employ atomic force microscopy (AFM), small-angle X-ray scattering (SAXS), small-angle neutron scattering (SANS), static light scattering (SLS), dynamic light scattering (DLS), osmometry, and multi-dimensional nuclear magnetic resonance (NMR) relaxometry and diffusometry. A goal of our basic tissue-sciences research is to develop understanding and tools that can be translated from bench-based quantitative methodologies to the bedside.
Our tissue sciences activities dovetail with our basic and applied research in quantitative imaging, which is intended to generate measurements and maps of intrinsic physical quantities, including diffusivities, relaxivities, exchange rates, etc., rather than the qualitative images conventionally used in radiology. At a basic level, our work is directed toward making key invisible biological structures and processes visible. Our quantitative imaging group uses knowledge of physics, engineering, applied mathematics, imaging and computer sciences, as well as insights gleaned from our tissue-sciences research to discover and develop novel quantitative imaging biomarkers that can detect changes in tissue composition, microstructure, or microdynamics with high sensitivity and specificity. The ultimate translational goal is to assess normal and abnormal developmental trajectories, diagnose childhood diseases and disorders, and characterize degeneration and trauma (such as TBI). Primarily, we use MRI as our imaging modality of choice because it is so well suited to many applications critical to the NICHD mission; it is noninvasive, nonionizing, requires (in most cases) no exogenous contrast agents or dyes, and is generally deemed safe and effective for use with mothers, fetuses, and children in both clinical and research settings.
One of our technical translational goals has been to transform clinical MRI scanners into scientific instruments capable of producing reproducible, highly accurate, and precise imaging data with which to measure and map useful imaging biomarkers for various clinical applications, including single scans, longitudinal and multi-site studies, personalized medicine, and genotype/phenotype correlation studies, as well as for populating imaging databases with high-quality normative data. From a more basic perspective, another goal has been to apply our various MRI tools and methodologies to advance the field of neuroscience, providing new means and methods to explore brain structure/function relationships.
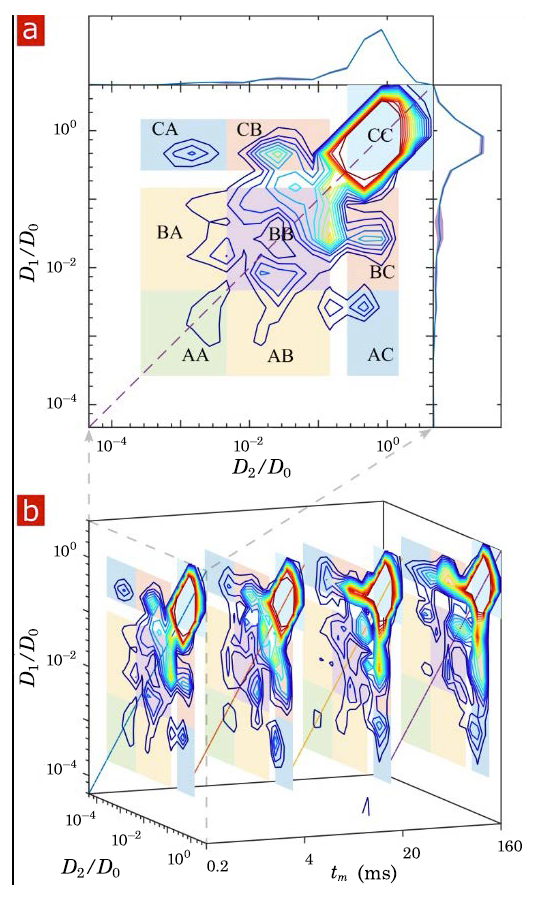
Figure 1. Diffusion Exchange Spectroscopy (DEXSY) reveals water exchange dynamics at sub-cellular length scales and sub-millisecond time scales.
The figure shows the exchange of water molecules between and among distinct water pools in neural tissue at sub-cellular length scales and sub-millisecond time scales. We developed means to perform DEXSY experiments at finer length and time scales than previously thought possible and are relevant to many cellular and subcellular processes. Peaks along the diagonal lines show water populations in different compartments, but as tm, the “mixing time,” increases, some water molecules migrate into different compartments, where their diffusivity may be different. The process is observable as the buildup of off-diagonal peaks. The rate at which water exchanges between the different water compartments or pools can be determined by studying the various water fractions and their evolution with respect to mixing time.
In vivo MRI histology
The most mature in vivo MRI histological technology that we invented, developed, and clinically translated is Diffusion Tensor MRI (DTI), by which we measure and map D, a diffusion tensor of water within an imaging volume. Information derived from this quantity includes white-matter fiber-tract orientation, the orientationally averaged mean apparent diffusion constant (mADC), and other intrinsic scalar (invariant) quantities. Such imaging parameters have been used by radiologists and neuroscientists as non-invasive quantitative histological ‘stains.’ These MRI images are obtained by probing endogenous tissue water in vivo without requiring any exogenous contrast agents or dyes. The mADC is used to identify ischemic areas in the brain during acute stroke and to follow cancer patients’ responses to therapy. Our measures of diffusion anisotropy (e.g., the fractional anisotropy or FA) are also widely used to follow changes in normally and abnormally developing white matter and in many other applications, such as brain white-matter visualization. Our group also pioneered the use of fiber direction–encoded color (DEC) maps to display the orientation of the main association, projection, and commissural white matter pathways in the brain. To assess anatomical connectivity among various cortical and deep-brain gray-matter areas, we also developed DTI “Streamline” Tractography, which is used to brain track white-matter fibers to help establish “anatomical connectivity” and by neuroradiologists and neurosurgeons to plan brain surgeries so that they can spare ‘eloquent’ areas of the brain. Collectively, these advances helped inspire several large federally funded research initiatives, including the NIH Human Connectome Project (HCP) and the NIH Brain Initiative.
More recently, we invented and developed a family of advanced in vivo diffusion MRI methods to measure fine-scale microstructural features of axons and fascicles, which otherwise could only be assessed using laborious ex vivo histological methods (i.e., “microstructure imaging”). We have been developing efficient means for performing “k- and q-space MRI” in the living human brain, such as “Mean Apparent Propagator” (MAP) MRI, an approach that can detect subtle microstructural and architectural features in both gray and white matter at micron-scale resolution, several orders of magnitude smaller than the typical MRI voxel size. MAP-MRI also subsumes DTI, as well as providing a bevy of new in vivo quantitative ‘stains’ or biomarkers to measure and map. We also developed a family of diffusion MRI methods to ‘drill down into the voxel’ to measure features such as average axon diameter (AAD) and axon-diameter distribution (ADD) within and along large white-matter pathways, dubbing them CHARMED and AxCaliber MRI, respectively. After careful validation studies, we reported the first in vivo measurement of ADDs within the rodent corpus callosum. The ADD is functionally important, given that axon diameter is a critical determinant of axon or nerve conduction velocity and therefore the rate at which information flows along axon bundles, and helps determine the latencies or time delays between and among different brain areas. We then developed a companion mathematical theory to explain the observed ADDs in different fascicles, suggesting that they represent a trade-off between maximizing information flow and minimizing metabolic demands. We also developed novel multiple pulsed-field gradient (mPFG) methods and demonstrated their feasibility in vivo on conventional clinical MRI scanners as a further means to extract quantitative features in the central nervous system (CNS) such as the AAD and other features of cell size and shape.
Although gray matter appears featureless in DTI brain maps, its microstructure and architecture are rich and varied throughout the brain, not only along the brain's cortical surface, but also within and among its various cortical layers and within deep gray-matter regions. To target this tissue, we have been developing several noninvasive, in vivo methods to measure unique features of cortical gray-matter microstructure and architecture that are visible in electron microscopy (EM) applications but currently invisible in conventional MRI. One example is diffusion tensor distribution (DTD) MRI. One of our long-term goals is to ‘parcellate’ or segment the cerebral cortex in vivo into its approximately 500 distinct cyto-architechtonic areas using noninvasive imaging methods. To this end, we are developing advanced MRI sequences to probe correlations among microscopic displacements of water molecules in the cortex as well as sophisticated mathematical models to infer distinguishing microstructural and morphological features of gray matter. We also pioneered several promising multi-dimensional MRI relaxometry and diffusometry-based methods to study water mobility and exchange in gray and white matter. We believe that these will eventually be translated to the clinic to help identify changes in normal and abnormal development, as well as inflammation and trauma.
Quantitative MRI biomarkers for pediatric applications
MRI is considered safer than X-ray–based methods, such as computed tomography (CT), for scanning fetuses, infants, and children. However, clinical MRI still lacks the quantitative character of CT. However, the scope of conventional MRI applications is limited to revealing either gross morphological features or focal abnormalities, which result in regional differences in signal intensities within a given tissue. Clinical MRI also often lacks biological specificity necessary for developing robust and reliable imaging “biomarkers.” In particular, MRI assessment of normal brain development and developmental disorders has benefited greatly from the introduction of “quantitative” clinical MRI techniques, with which one obtains maps of meaningful intrinsic physical quantities or chemical variables that can be measured in physical units and compared among different tissue regions, in individual subjects, and within longitudinal and cross-sectional studies. Quantitative MRI methods, such as DTI, also increase sensitivity, providing a basis for monitoring subtle changes that occur, e.g., during the progression or remission of disease, by comparing measurements in a single subject with normative values obtained from a healthy population. Quantitative MRI methods should continue to advance “precision medical imaging” studies, in which MRI phenotypic and genotypic data can be meaningfully melded and used for improved diagnosis and prognosis assessments.
To advance our quantitative imaging activities, we developed algorithms that generate a continuous, smooth approximation to the discrete, noisy, measured DTI field data so as to reduce noise and allow us to implement Streamline Tractography more reliably. We proposed a novel Gaussian distribution for the tensor-valued random variables that we use to design optimal DTI experiments and interpret their results. In tandem, we developed non-parametric empirical (e.g., Bootstrap) methods to determine the statistical distribution of DTI–derived quantities in order to study, e.g., the inherent variability and reliability of computed white-matter trajectories. Such parametric and non-parametric statistical methods enabled us to apply powerful hypothesis tests to assess the statistical significance of findings in a wide range of important biological and clinical applications that were tested using ad hoc statistical methods. We are also developing novel methods to register different brain volumes and to generate group-average DTI data or atlases from various subject populations based on the Kullback-Leibler divergence. However, much work remains to be done in order to address and remedy MRI artifacts so as to permit one to draw statistically significant and unbiased inferences from clinical DTI data obtained in longitudinal and multi-center studies, and particularly in single-subject studies.
We carried out key clinical studies that utilize novel quantitative MRI acquisition and analysis methods and whose aim is to improve accuracy and reproducibility in diagnosis and to detect and follow normal and abnormal development. One early example is the NIH Study of Normal Brain Development, jointly sponsored by the NICHD, NIMH, NINDS, and NIDA. This multi-center consortium, initiated in 1998, was intended to advance our understanding of normal brain development in typical healthy children and adolescents. The Brain Development Cooperative Group (http://www.brain-child.org/brain_group.html), created by this funding mechanism, is still actively publishing papers, primarily by mining the rich high-quality MRI data, many of which our lab processed, vetted, and uploaded, serving as the DTI Data-Processing Center (DPC) in this interdisciplinary project. The processed DTI data collected from the project were uploaded into a database accessible to interested investigators, and made publicly available through the National Database for Autism Research (NDAR; http://ndar.nih.gov). Carlo Pierpaoli, who spearheaded this work, continues to support, update, and disseminate the processing and analysis software called “TORTOISE,” that grew out of this effort and which can be downloaded from http://www.tortoisedti.org.
Traumatic Brain Injury (TBI) represents a significant public health challenge for our pediatric population, but also for young men and women in the military. Our involvement in TBI research, particularly in trying to detect mild TBI (mTBI), has continued to expand in partnership with various Department of Defense (DoD) affiliates. Diffusion MRI provides essential information to aid in the assessment of TBI, but it lacks sufficient specificity. Because of subtle changes seen in TBI, new sensitive and specific quantitative imaging protocols are required. To this end, we developed a DTI data–processing pipeline in order to improve the accuracy and reproducibility of MAP–MRI findings, and, in collaboration with scientists at the DoD Center for Neuroscience and Regenerative Medicine (CNRM), performed the first normative MAP–MRI studies, as well as applied this new and powerful method to detect tissue damage in brains of individuals who have suffered mild or moderate TBI. To enable this application, we are extending our NICHD TORTOISE pipeline to analyze MAP–MRI data. We are now employing multi-dimensional MRI methods to study the etiology of various type of TBI, in collaboration with the Neuropathology Core of the CNRM.
We are also collaborating with Sara Inati, who has a dedicated clinical research program to study and diagnose focal epilepsy, a devastating disorder that is difficult to detect using conventional radiological methods. We are developing a bevy of new MRI–based methods that we believe may reveal pathological microstructural features in the disorder, for example, in cortical dysplasias.
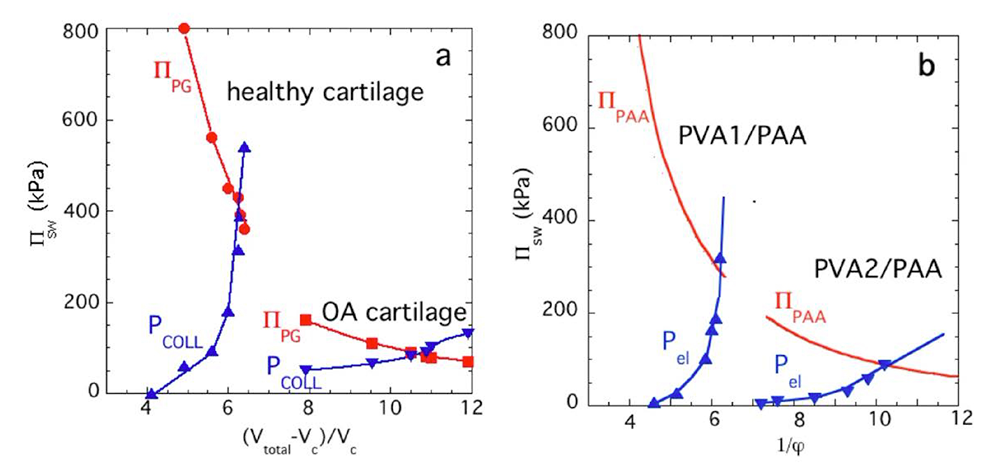
Figure 2. Load-bearing behavior of a novel composite biomimetic cartilage hydrogel juxtaposed with that of excised human cartilage
The figure shows the mechanical behavior of real human cartilage juxtaposed with that of our new biomimetic cartilage construct. Our composite hydrogel contains a microgel component that mimics the proteoglycan (PG) phase, which imbibes water, and a polymer network that mimics the fibrous Type II collagen, which confines PG swelling. The balance between the swelling pressure and tensile stresses restraining it allow the two soft and “squishy” constituents to swell much the way a dialysis bag filled with polymers, swells into a tough and stiff material. Our synthetic cartilage is as strong and stiff as real cartilage and also can be used as a model to mimic the loss of tissue stiffness in osteoarthritis. The novel marriage of polymer physics and biomaterials provides a new conceptual framework for developing composite functional tissue-engineered cartilage constructs and other extracellular matrices.
Biopolymer physics: water-ion-biopolymer interactions
Water-ion-biopolymer interactions play a myriad of roles in biology. Our primary objective in this project is to understand such interactions in the biological milieu. Remarkably, little is understood about their physical underpinnings, particularly in their physiological ionic strength regime, despite their crucial role.
To determine the effect of ions on the structure and dynamics of key biopolymers, we developed a multi-scale experimental framework by combining macroscopic techniques (osmotic swelling pressure measurements, mechanical measurements) with scattering methods (e.g., SANS and SAXS), which probe the structure and interactions over a broad range of length and time scales. Macroscopic swelling pressure measurements provide information on the overall thermodynamic response of the system, while SANS and SAXS allow us to investigate biopolymers at molecular and supramolecular length scales and to quantify the effect of changes in the environment (e.g., ion concentration, ion valance, pH, temperature) on the structure and interactions among biopolymers, water, and ions. Studies carried out on well-defined model systems that mimic essential features of tissue provide important insights that cannot be obtained from experimental studies made on biological systems. Mathematical models based on well-established polymer physics concepts and molecular dynamics approaches make it possible to design and analyze fundamental experiments, which quantify and explain aspects of tissue behavior in order to gain insight into the underlying molecular mechanism that governs key aspects of tissue structure-function relationships.
Better understanding of the structure and interactions among tissue components is also necessary to design and develop models and phantoms that mimic tissue behavior. Biomimetic phantoms with well-controlled physical properties (osmotic, mechanical, relaxation, etc.) are critically important to validate our quantitative MRI applications, from bench to bedside in our imaging studies. We produced novel diffusion MRI phantoms, which we use to calibrate MRI scanners, specifically to assure the quality of the imaging data and to assess scanner performance on an on-going basis. Our U.S. Patent for a “Phantom for diffusion MRI imaging” is now enabling quantitative diffusion MRI studies to be performed at many different sites. Our colleagues at NIST in Boulder, Colorado, have incorporated our PVP polymer into their own diffusion MRI NIST standard. The technology is also being promulgated commercially e.g., by by High Precision Devices, Inc. We also developed a variety of NMR and MRI phantoms that possess various salient features of cell or tissue systems, providing ‘ground truth’ data with which to test the validity of our models and experimental designs.
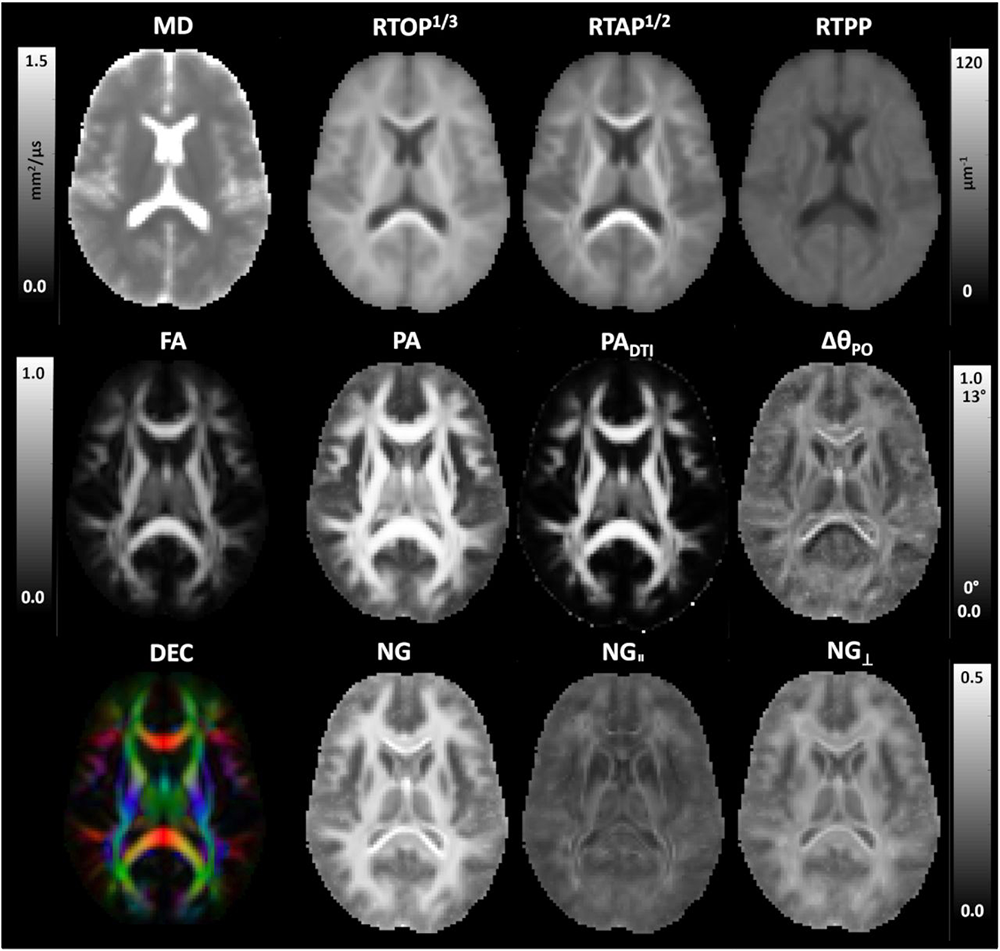
Figure 3. Various quantitative microstructural “stains” obtained from Mean Apparent Propagator (MAP) MRI from cohort of healthy human subjects
Microstructural MAP-MRI parameters were computed from a template (or atlas) of mean net displacement propagators measured in a cohort of 12 healthy volunteers: diffusion tensor imaging (DTI) parameters, mean diffusivity (MD), fractional anisotropy (FA), and direction encoded color (DEC) map; along with MAP microstructural parameters: return-to-origin probability (RTOP), return-to-axis probability (RTAP), return-to-plane probability (RTPP), propagator anisotropy (PA), Gaussian propagator anisotropy (PADTI), non-Gaussian anisotropy (ΘPO), non-Gaussianity (NG), axial non-Gaussianity (NG||), and radial non-Gaussianity (NG⊥). These quantities highlight different microstructural features of gray and white matter and are obtained without any exogenous contrast agents or dyes, and without using ionizing radiation.
Measuring and mapping functional properties of extracellular matrix (ECM)
Extracellular matrix (ECM) is present in every tissue and performs a myriad of roles in determining normal and abnormal tissue and organ function. We study interactions among the main ECM components, using cartilage as a model system. In cartilage ECM, collagen (type II) is organized into fiber bundles that form a network that entraps the major proteoglycan (PG), a bottlebrush-shaped aggrecan. The biomechanical behavior of cartilage and other ECMs reflects their molecular composition and microstructure, which change during development, disease, degeneration, and aging. To determine tissue structure/function relationships, we measure various physical/chemical properties of ECM tissues and tissue analogs at different length and time scales, using a variety of complementary static and dynamic experimental techniques, e.g., osmometry, SANS, SAXS, neutron spin-echo (NSE), SLS, DLS, and AFM. Understanding the physical and chemical mechanisms affecting cartilage swelling (hydration) is essential to predicting its load-bearing ability, which is mainly governed by osmotic and electrostatic forces. To quantify the effect of hydration on cartilage properties, we previously developed a novel tissue micro-osmometer to perform precise and rapid measurements on small tissue samples (less than 1 microgram) as a function of the equilibrium water activity (vapor pressure). We also make osmotic pressure measurements to determine how the individual components of cartilage ECM (e.g., aggrecan and collagen) contribute to the total load-bearing capacity of the tissue. We also demonstrated that aggrecan-hyaluronic aggregates self-assemble into microgels, contributing to improved dimensional stability of the tissue and to its lubricating ability. We also found that aggrecan is highly insensitive to changes in the ionic environment, particularly to calcium ions, which is critically important to maintaining the tissue's mechanical integrity and to allowing aggrecan to serve as a calcium ion reservoir in cartilage and bone.
To model cartilage ECM, we recently invented and developed a new biomimetic material consisting of polyacrylic acid (PAA) microgel particles dispersed and embedded within a polyvinyl alcohol (PVA) gel matrix. In this novel composite hydrogel, PAA mimics the proteoglycan (i.e., hyaluronic-aggrecan complexes), while PVA mimics the fibrous collagen network entrapping them. Remarkably, the PVA/PAA biomimetic model system reproduces not only the shape of the cartilage swelling pressure curves, but also the numerical stiffness values reported for healthy and osteoarthritic human cartilage samples. Systematic studies made on these model composite hydrogels should yield invaluable insights into the effects of various macromolecular factors (matrix stiffness, swelling pressure, fixed-charge density, etc.) on the tissue's macroscopic mechanical/swelling properties, and ultimately the origin of its load-bearing and lubricating abilities.
We are now attempting to translate this critical tissue-science understanding of the structure-function relationships of ECM components to develop and design novel non-invasive MRI methods, with the aim of inferring ECM composition, patency, and functional properties in vivo. Our goal is to use MRI for early diagnosis of diseases of cartilage and other tissue and organs, as well as to provide a means for following normal and abnormal ECM development, which entails making ‘invisible’ components of ECM, (e.g., collagen and PGs) ‘visible,’ and then using our understanding of biopolymer interactions to predict functional properties of the composite tissue, such as its load-bearing ability. One major obstacle is that protons, i.e., hydrogen atoms, bound to immobile species (e.g., collagen) are largely invisible using conventional MRI. However, magnetization exchange (MEX) MRI (as well as other methods) make it possible to detect the bound protons indirectly by transferring their magnetization to the free water protons surrounding them. It also enables us to estimate collagen content in tissue quantitatively. In pilot studies with Uzi Eliav and Ed Mertz, we applied the new MEX MRI method to determine the concentration and distribution of the main macromolecular constituents in bovine femoral-head cartilage samples. The results were qualitatively consistent with those obtained by histological techniques, such as high-definition infrared (HDIRI) spectroscopic imaging. The work was originally supported by a DIR Director’s Award to our collaborators Sergey Leikin and Edward Mertz, which they are continuing to pursue collaboratively. Our novel approach has the potential to map tissue structure and functional properties in vivo and noninvasively. In tandem, we are now developing continuum models of cartilage and cartilage ECM analogs in order to explain and interpret our experimental findings, develop and test novel hypotheses, and predict the behavior of our model system under different experimental conditions.
We also recently began using several novel one-sided NMR methodologies to study water relaxation, diffusion, and exchange behaviors in ECM as a means to infer and characterize its critical functional properties. Our specialized NMR scanner can probe layered media, such as cartilage, using ultra-thin slices, almost as thin as a confocal microscope provides. However, optical imaging is not a viable option, given that these tissues are turbid.
Patents
- Benjamini D, Basser PJ. Multi-dimensional spectroscopic NMR and MRI using marginal distributions. USPTO Patent No. WO2018031942-A1 2018;N/A.
- Horkay F, Pierpaoli C, Basser PJ. Phantom for diffusion MRI imaging. USPTO Patent No. 10,078,124 2018;N/A.
Additional Funding
- “Development of Bench and Pre-Clinical MRI Methods to Assess Glymphatic Clearance in the Living Brain.” 308811-4.01-60855, (CNRM-89-9237), which is under the joint auspices of the NIH, DoD, CNRM, and USUHS.
- In vivo Brain Network Latency Mapping.” NIH BRAIN Initiative grant 1-R24-MH-109068-01.
- “Connectome 2.0: Developing the next generation human MRI scanner for bridging studies of the micro-, meso- and macro-connectome.” NIH BRAIN Initiative-funded 1U01EB026996-01.
- “MRI methods aimed at the detection of pathologies involving myelin, collagen and amyloid plaques.” Bi-National Science Foundation (BSF) grant; 2013-2018, #2013253.
- “Neuroradiology/Neuropathology Correlation/Integration Core.” 309698-4.01-65310, (CNRM-89-9921).
- “Localization of Epileptogenic Foci using Diffusion Weighted MRI.” Bench-to-Bedside (BtB) Award, NIH Director’s Challenge Innovation Grant, NIH IRP.
Publications
- Bai R, Springer C, Plenz D, Basser P. Brain active transmembrane water cycling measured by MR is associated with neuronal activity. Magn Reson Med 2019;81(2):1280-1295.
- Williamson NH, Ravin R, Cai TX, Benjamini D, Falgairolle M, O'Donovan MJ, Basser PJ. Real-time measurement of diffusion exchange rate in biological tissue. J Magn Reson 2020;317:106782.
- Benjamini D, Basser P. Water mobility spectral imaging of the spinal cord: Parametrization of model-free Laplace MRI. Magn Reson Imaging 2019;56:187-193.
- Horkay F, Basser PJ. Composite hydrogel model of cartilage predicts its load-bearing ability. Sci Rep 2020;10:8103.
- Mussel M, Basser PJ, Horkay F. Effects of mono- and divalent cations on the structure and thermodynamic properties of polyelectrolyte gels. Soft Matter 2019;15(20):4153-4161.
- Horkay F. Effect of the ionic environment on the supramolecular structure and thermodynamics of DNA gels. Macromol Symp 2019;385:1800199.
Collaborators
- Ruliang Bai, PhD, Zhejiang University, Hangzhou, China
- John Butman, MD, PhD, Radiology and Imaging Sciences, Clinical Center, NIH, Bethesda, MD
- Emilios Dimitriadis, PhD, Division of Bioengineering and Physical Science, NIBIB, Bethesda, MD
- Jack Douglas, PhD, NIST, Gaithersburg, MD
- Uzi Eliav, PhD, Tel Aviv University, Tel Aviv, Israel
- Dario Gasbarra, PhD, University of Helsinki, Helsinki, Finland
- Erik Geissler, PhD, CNRS, Université Joseph Fourier de Grenoble, Grenoble, France
- Mark Hallett, MD, PhD, Human Motor Control Section, NINDS, Bethesda, MD
- Iren Horkayne-Szakaly, MD, Joint Pathology Center, Armed Forces Institute of Pathology, Washington, DC
- Sara Inati, MD, Electroencephalography (EEG) Section, NINDS, Bethesda, MD
- Sergey Leikin, PhD, Section on Physical Biochemistry, NICHD, Bethesda, MD
- Edward L. Mertz, PhD, Section on Physical Biochemistry, NICHD, Bethesda, MD
- Gil Navon, PhD, Tel Aviv University, Tel Aviv, Israel
- Dzung Pham, PhD, CNRM Imaging Core, Henry M. Jackson Foundation, Bethesda, MD
- Carlo Pierpaoli, MD, PhD, Section on Quantitative Medical Imaging, NIBIB, Bethesda, MD
- Dietmar Plenz, PhD, Section on Critical Brain Dynamics, NIMH, Bethesda, MD
- Tom Pohida, MS, Signal Processing and Instrumentation Section, CIT, NIH, Bethesda, MD
- Randall Pursley, Signal Processing and Instrumentation Section, CIT, NIH, Bethesda
- Evren Özarslan, PhD, Linköping University, Linköping, Sweden
- Joelle Sarlls, PhD, In Vivo NMR Center, NINDS, Bethesda, MD
- Brain Development Cooperative Group, Various
Contact
For more information, email pjbasser@helix.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/basser.