Mechanisms of Synapse Assembly and Homeostasis

- Mihaela Serpe, PhD, Head, Section on Cellular Communication
- Peter Nguyen, Biological Laboratory Technician
- Tae Hee Han, PhD, Staff Scientist
- Saumitra Dey Choudhury, PhD, Visiting Fellow
- Wen Chieh Hsieh, PhD, Visiting Fellow
- Tho Huu Nguyen, PhD, Visiting Fellow
- Rosario Vicidomini, PhD, Visiting Fellow
- Thomas Brody, PhD, Special Volunteer
In our research we strive to understand the mechanisms of synapse assembly, plasticity, and homeostasis. The chemical synapse is the fundamental communication unit connecting neurons in the nervous system to one another and to non-neuronal cells and whose purpose is to mediate rapid and efficient transmission of signals across the synaptic cleft. Synaptic transmission forms the basis of the biological computations that underlie and enable our complex behavior. Crucial to this function is the ability of a synapse to change its properties, so that it can optimize its activity and adapt to the status of the cells engaged in communication and/or to the larger network comprising them. Consequently, synapse development is a highly orchestrated process coordinated by intercellular communication between the pre- and postsynaptic compartments and by neuronal activity itself. Our long-term goal is to elucidate the molecular mechanisms that regulate formation of functional synapses during development and which fine-tune them during plasticity and homeostasis. We focus on four key processes in synaptogenesis: (1) trafficking of components to the proper site; (2) organizing those components to build synaptic structures; (3) maturation of the synapse to optimize its activity; and (4) homeostatic mechanisms that restore synapse activity after various perturbations in the system. We address the molecular mechanisms underlying these processes using a comprehensive set of approaches that include genetics, biochemistry, molecular biology, super-resolution imaging, and electrophysiology recordings in live animals and reconstituted systems.
Because of its many advantages, we study these events in a powerful genetics system, Drosophila melanogaster, and use the neuromuscular junction (NMJ) as a model for glutamatergic synapse development and function. The fact that individual NMJs can be reproducibly identified from animal to animal and are easily accessible for electrophysiological and optical analysis makes them uniquely suited for in vivo studies on synapse assembly, growth, and plasticity. In addition, the richness of genetic manipulations that can be performed in Drosophila permits independent control of individual synaptic components in distinct cellular compartments. Importantly, the fly NMJ relies entirely on kainate-type receptors, a family of ionotropic glutamate receptors that impact synaptic transmission and neuronal excitability in the mammalian central nervous system but remain poorly understood. The Drosophila NMJ can thus be used to analyze and model defects in the structural and physiological plasticity of glutamatergic synapses, which are associated with a variety of human pathologies, from learning and memory deficits to autism. The similarity in architecture, function, and molecular machinery supports the notion that studying the assembly and development of fly glutamatergic synapses will shed light on their human counterparts.
Neto, an auxiliary subunit for glutamate receptors, is essential for synapse development.
Many neurological disorders are linked to defects in synaptogenesis; however, the initial clustering functions critical for the synaptic recruitment and stabilization of neurotransmitter receptors are poorly understood. At vertebrate or insect NMJs, prior to motor neuron arrival at its target muscle, the neurotransmitter receptors form small, nascent clusters on the muscle, which are distributed in the vicinity of future synaptic sites. Motor neuron arrival triggers the formation of large synaptic receptor aggregates and promotes expression of more receptors to permit synapse maturation and growth. In flies as in vertebrates, the neurotransmitter receptor clusters interact with the local cytoskeleton and other synaptic structures to maintain local density, which involves solving three fundamental problems common to all chemical synapses: (1) trafficking the components to the proper site; (2) organizing them to build synaptic structures; and (3) maturation and homeostasis of the synapse to optimize its activity. Recent advances, particularly from vertebrate ionotropic glutamate receptor (iGluR) biology, reveal that the solution to such problems entirely depends on the activity of a rich array of auxiliary subunits that associate with the receptors. These highly diverse transmembrane proteins associate with iGluRs at all stages of the receptor life-cycle and mediate the delivery of receptors to the cell surface, their subcellular distribution, synaptic recruitment, their endocytosis and turnover, association with various post-synaptic density (PSD) scaffolds, and importantly, their channel properties. iGluRs assembled from different subunits have strikingly different biophysical properties; their association with different auxiliary subunits increases this diversity even further.
The Drosophila NMJ utilizes at least six kainate receptor (KAR) subunits, which form two distinct postsynaptic complexes (type-A and type-B) that co-exist within individual postsynaptic densities (PSDs) and enable NMJ functionality and plasticity, and a presynaptic complex containing the presynaptic KAR KaiRID, which modulates basal neurotransmission. The postsynaptic KARs are heterotetrameric complexes composed of three shared subunits, GluRIIC, GluRIID, and GluRIIE, as well as either GluRIIA (type-A receptors) or GluRIIB (type-B). The shared subunits are required for animal viability as they are essential for iGluR synaptic recruitment and therefore NMJ assembly and function. Our previous studies identified Drosophila Neto as an obligatory subunit of fly NMJ iGluR complexes. Neto belongs to a family of highly conserved auxiliary proteins that share an ancestral role in the formation and modulation of glutamatergic synapses. Vertebrate Neto1 and Neto2 and Caenorhabditis elegans Neto/SOL-2 were recently shown to modulate the properties of selective iGluRs, mostly KARs. Neto1/Neto2 double knockout mice have defects in long-term potentiation and in learning and memory, but the underlying mechanisms are extremely difficult to study owing to the low abundance of such channels and the small currents they elicit. In contrast, we found that Drosophila utilizes Neto and KARs at the NMJ, a synapse essential for viability. Using live imaging we showed that Neto clusters at nascent NMJs at the time when iGluRs begin to accumulate and cluster. Similar to mutants lacking essential, shared iGluR subunits, netonull mutants are completely paralyzed and die as embryos, with the iGluRs scattered as small aggregates, away from the neuronal arbor. Importantly, Neto does not cluster at synaptic locations in the absence of iGluRs, indicating that Neto and iGluRs depend on each other for trafficking and stabilization at synaptic sites. Our studies demonstrate that Neto engages the iGluRs on the muscle membrane and traffic together to synaptic sites where they form clusters. By controlling the clustering and trafficking of functional iGluR complexes, Neto directly controls synapse assembly, organization and maintenance of PSDs, and synapse functionality.
Neto–mediated intracellular interactions sculpt the postsynaptic iGluR fields.
The Neto proteins are multi-domain transmembrane proteins with two extracellular CUB (for complement C1r/C1s, UEGF, BMP-1) domains followed by an LDLa (low-density lipoprotein receptor domain class A) motif. CUB domains are BMP–binding, protein-interaction domains that could promote aggregation. Drosophila neto encodes two isoforms, Neto-α and Neto-β, with different cytoplasmic domains, generated by alternative splicing. The cytoplasmic domains, both rich in putative phosphorylation motifs and docking sites, are highly divergent among Neto proteins across species, presumably reflecting cell/tissue specific roles. To characterize the functional domains of Neto, we generated truncated Neto variants and tested their cellular distribution and ability to rescue Neto function during development. In previous studies we had found that the extracellular part of Neto is required for apical targeting as well as for clustering of Neto/iGluR complexes at the NMJ. Thus, muscle expression of a Neto variant with no intracellular domain (Neto-ΔCTD) can rescue the iGluRs recruitment in netonull mutants. We found that Neto activities are restricted by an inhibitory prodomain, which must be removed by Furin-mediated proteolysis. When the prodomain cleavage is blocked, Neto is properly targeted to the muscle membrane and engages the iGluR complexes in vivo but fails to enable the incorporation of iGluRs in stable synaptic clusters. The recruitment of PSD components is partly attributable to Neto–mediated intracellular interactions.
Neto-β is the predominant Neto isoform at the larval NMJ [Reference 1]. Our developmental studies indicate that Neto-β controls the synaptic recruitment of iGluRs and of other postsynaptic components, such as P21–activating kinase (PAK), an important PSD component previously implicated in the stabilization of type-A receptors at postsynaptic sites. Type-A and type-B glutamate receptor complexes differ in their composition, function, and mechanisms of trafficking to the synapses. Using a neto-β allelic series, we found that Neto-β uses its cytoplasmic domain as an organizing platform to recruit iGluRs and PSD proteins that stabilize the synaptic type-A receptors, thus sculpting the postsynaptic composition.
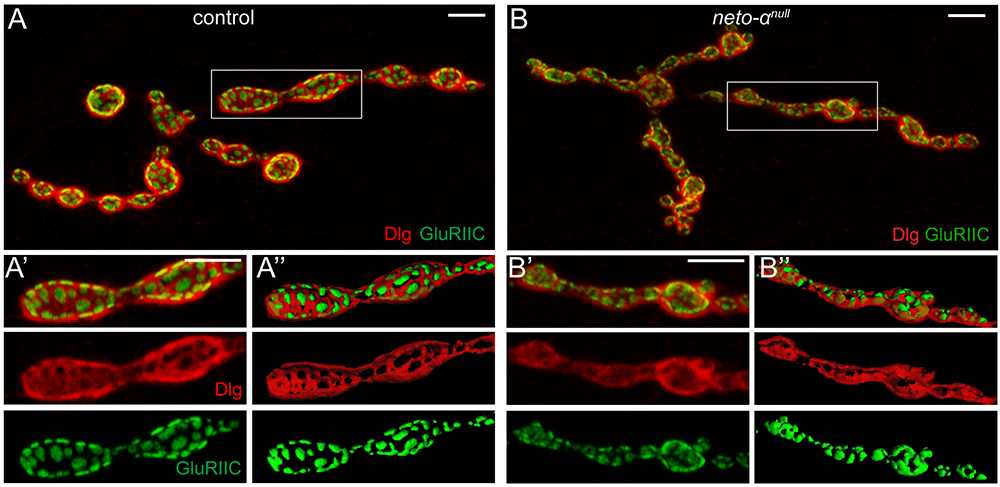
Figure 1. Neto-α limits the postsynaptic receptor field.
A–B". Confocal images and 3D reconstitution of NMJ boutons labeled with Discs large (Dlg - red) and GluRIIC (green). In control animals, Dlg–positive staining abuts GluRIIC–marked PSDs. The borders between Dlg and GluRIIC are blurred in neto-αnull boutons (B-B").
Neto-α represents less than 10% of the total Neto pool at the larval NMJ. We found that loss of Neto-α has no detectable effect on postsynaptic iGluR levels or the relative type-A:type-B receptor ratio but it does influence the iGluR receptor field [Reference 1]. In flies, the PSD-95 ortholog Discs Large (Dlg) flanks the PSD/iGluR fields, marked by GluRIIC, an obligatory subunit of the postsynaptic iGluR complexes (Figure 1). The PSD/Dlg boundaries are well defined in control boutons but are no longer recognizable at neto-αnull NMJs. Furthermore, the 3D reconstructions of the boutons showed no overlap between GluRIIC and Dlg signals in controls but significant overlap in neto-αnull mutants. 3D-structured illumination microscopy (3D-SIM) and serial electron microscopy analyses also captured significantly enlarged (by 30%) receptor fields at neto-αnull NMJs in (Figure 2). Muscle overexpression of a neto-α transgene fully rescued the PSD size of neto-αnull synapses. Thus, postsynaptic Neto-α limits the postsynaptic receptor fields.
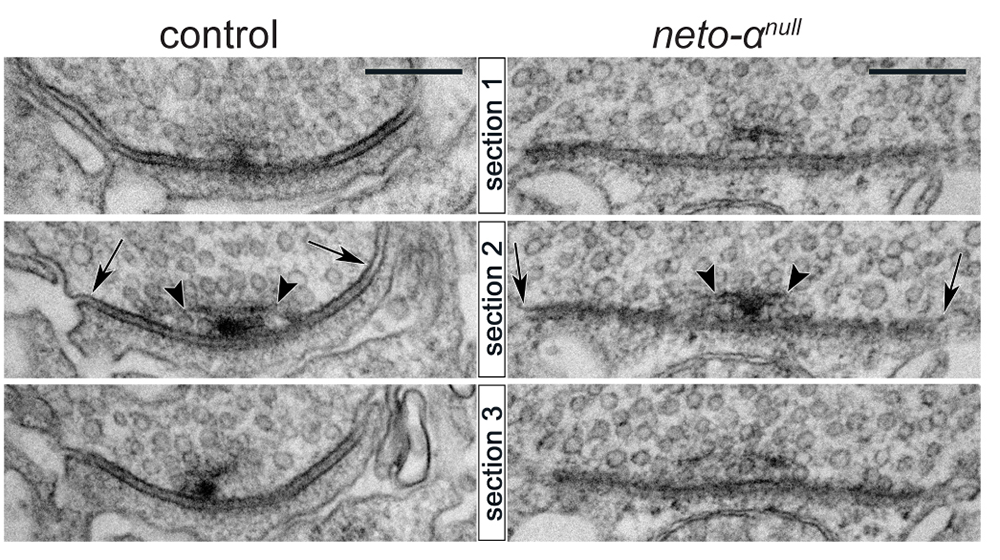
Figure 2. Enlarged postsynaptic densities at neto-αnull NMJs
Serial sections of electron micrographs of single postsynaptic densities (PSD) in control and neto-αnull boutons. The longest diameters detectable in serial sections for each PSD or T-bar structure are indicated by arrows and arrowheads, respectively.
Presynaptic Neto-α and KaiRID control basal neurotransmission.
Loss-of-function and genetic epistasis experiments uncovered an unexpected role for Neto-α in the control of basal neurotransmission. Intracellular recordings of spontaneous miniature excitatory junctional potentials (mEJPs) and evoked excitatory junctional potentials (EJPs) from neto-αnull muscle indicate normal miniature amplitudes, a result consistent with normal iGluR levels at neto-αnull NMJs. However, neto-αnull animals showed EJP amplitudes reduced by 46% [Reference 1]. Knockdown of Neto-α in the motor neurons but not in the muscle induced similarly reduced EJP amplitudes; conversely, neuronal but not muscle expression of neto-αnull transgenes rescued the EJP amplitudes at neto-αnull NMJs. The data indicate that presynaptic Neto-α is required in the motor neurons to ensure normal levels of basal neurotransmission.
Given that the presynaptic KaiRID–containing complex was recently implicated in the control of basal neurotransmission, we examined whether Neto-α modulates KaiRID synaptic distribution and function. We found that neto-αnull and KaiRIDnull single and double mutants have similar physiologic deficits. Also, neuronal expression of Neto-α and KaiRID can rescue the corresponding mutants, but cannot compensate for each other, indicating that Neto-α and KaiRID function together to control basal neurotransmission. We previously demonstrated that a minimal Neto variant called Neto-deltaCTD, which contains the highly conserved domains shared by Neto-α and Neto-β (the extracellular CUB domains, LDLa motif, and the transmembrane part) but has no intracellular C-terminal domain, is both required and sufficient for the synaptic recruitment and function of postsynaptic iGluRs. Interestingly, neuronal Neto-deltaCTD rescued basal neurotransmission at neto-αnull mutant NMJs. In unpublished studies, we found that Neto-α and Neto-deltaCTD increase the desensitization rates and open probability for the KaiRID channels, thus modulating the function of this auto-receptor.
Similar to the muscle, we found that Neto-α, not the iGluR subunits, appears to be the limiting component at presynaptic terminals. Neuronal overexpression of KaiRID has no effect on the baseline, whereas overexpression of Neto-α or Neto-deltaCTD proportionally increases the basal neurotransmission levels. Thus, the auto-receptor function and likely synaptic levels are tightly controlled by Neto-α.
Neto-α is an effector of synapse homeostasis.
In flies as in vertebrates, neuronal activity induces input-specific changes in the synaptic strength; at larval NMJ, the postsynaptic sensitivity is primarily modulated via synapse-specific recruitment of type-A (GluRIIA–containing) receptors. Robust homeostatic mechanisms keep synapses within an appropriate dynamic range, so that the evoked potentials measured in the muscle remain constant from embryo to third instar larvae; reduced postsynaptic sensitivities (i.e., reduced GluRIIA activity) trigger a compensatory increase in quantal content (QC), the number of vesicles released by the neuron, known as presynaptic homeostatic potentiation (PHP). To study this homeostatic response, we used two well-established paradigms: (1) chronic (developmental); and (2) acute (pharmacological) induction of PHP. Specifically, (1) removal of GluRIIA during development leads to reduced quantal size (mEJP) and triggers PHP (increased QC), a PHP response that is not detectable in neto-αnull;GluRIIA double mutants. Also, (2) application of sub-blocking concentrations of philanthotoxin (PhTx), a polyamine toxin derived from wasp venom, to semi-intact larval preparations triggers a fast reduction in quantal size and an increase in QC, so that basal neurotransmission recovers within minutes. PhTx reduces the quantal size at neto-αnull NMJs, but basal neurotransmission never recovers. Similar to above, the PHP deficits in neto-αnull mutants phenocopy those of KaiRIDnull mutants. However, neuronal overexpression of Neto-α rescues the PHP defects in neto-αnull;KaiRIDnull double or KaiRIDnull single mutants, indicating that Neto-α is both required and sufficient for the induction of the PHP response. Moreover, we found that Neto-deltaCTD cannot sustain any PHP response, indicating that the intracellular domain of Neto-α is critical for this function.
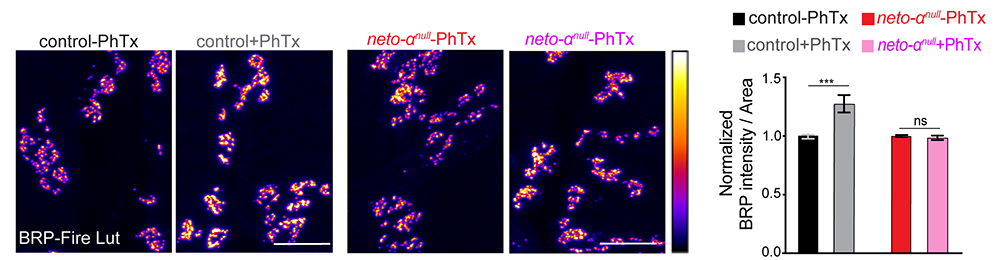
How does Neto-α enable such homeostatic compensatory response? We found no deficits in the presynaptic Ca2+ entry at neto-αnull–mutant NMJs. The absence of Neto-α does not alter the pool of release-ready presynaptic vesicles and therefore could not cause the observed reduced basal neurotransmission in animals with reduced postsynaptic sensitivity. However, we found that presynaptic active zone scaffold, Bruchpilot (Brp), undergoes a rapid synaptic accumulation upon pharmacological induction of PHP in control animals but not in neto-αnull mutants (Figure 3). The findings suggest that, in response to reduced postsynaptic sensitivity, Neto-α functions to swiftly mobilize Brp, which presumably enhances vesicle release and enables the compensatory response.
Figure 3. Impaired cytomatrix remodeling at neto-αnull synaptic terminals
Quantification of Bruchpilot (Brp) intensity following 10 min of vehicle or Philanthotoxin-343 (PhTx) treatment at control and neto-αnull NMJs. PhTx is an effective iGluR blocker. Brp is shown in Fire-lut. On the intensity scale, white represents peak intensity (20,000 arbitrary units). PhTx application triggers increased Brp signal intensity in control but not in neto-αnull boutons.
Our data demonstrate that the two major functions of Neto-α in the presynaptic compartment could be segregated and mapped to different domains: (1) the minimal Neto, or Neto-deltaCTD, which modulates basal neurotransmission, likely by modulating the KaiRID function; and (2) the intracellular part of Neto-α, which is both required and sufficient for the presynaptic homeostatic response. The limiting Neto-α appears to be recruited at synapses by the presynaptic KaiRID, a finding that challenges our current thinking that auxiliary subunits “assist” iGluRs and that provides an exquisite example of an auxiliary protein that performs a key synaptic function with assistance from iGluRs.
Differential subcellular distribution for Neto isoforms
Intriguingly, neuronal expression of Neto-β cannot rescue the basal neurotransmission and PHP deficits at neto-αnull NMJs, which is unexpected, because Neto-β contains all the domains of the “minimal Neto” (Neto-deltaCTD) and, at the least, should have rescued the basal neurotransmission levels. We examined the Neto-β–specific signals and found that neuronal overexpression of Neto-β did not induce accumulation along axons or at synaptic terminals; instead, Neto-β remained restricted to the somato-dendritic compartment. Current efforts focus on elucidating the protein motifs and the molecular pathways that block the trafficking of Neto-β through the axonal initiation segment and into the axonal compartment and restrict Neto-β to the somato-dendritic compartment.
Together, our studies provide strong evidence for Neto activities at presynaptic terminals. Previous work showed that presynaptic KARs regulate neurotransmitter release; however, the site and mechanism of action of presynaptic KARs have been difficult to pin down. Our structure-function analyses, together with the fact acute PHP induction occurs even when the axon is severed, indicate that Neto-α together with KaiRID, localizes at presynaptic terminals, where KaiRID could function as an auto-receptor.
Our current efforts focus on identifying proteins that interact with Neto both inside and outside the cell and provide critical activities at the developing NMJ, including: (1) iGluR–clustering; (2) iGluR recruitment and stabilization at PSDs; and (3) mediating PHP. To this end, we initiated several complementary screens: pull-down and mass-spectroscopy comparisons of proteins interacting with the intracellular domains of the fly Neto proteins (α and β); and a synthetic lethality screen.
Local BMP/BMPR complexes regulate synaptic plasticity.
Synaptic activity and synapse development are intimately linked, but our understanding of the coupling mechanisms is limited. In particular, how synapse activity status is monitored and communicated across the synaptic cleft remains poorly understood. Our previous studies uncovered a role for bone morphogenetic proteins (BMPs) in sensing the activity of post-synaptic receptors and relaying this information across the synaptic cleft.
At the Drosophila NMJ, BMP signaling is critical for NMJ growth, neurotransmitter release, and synapse plasticity and homeostasis. We discovered a completely novel BMP signaling modality that operates in conjunction with the canonical BMP pathway to ensure those functions. The canonical BMP pathway, triggered by muscle-derived Gbb binding to presynaptic BMP type-II receptor (BMPRII), Wishful thinking (Wit), and the BMPRIs Thickveins (Tkv) and Saxophone (Sax), induces accumulation of phosphorylated Smad (pMad) in motor-neuron nuclei and activates transcriptional programs with distinct roles in the structural and functional development of the NMJ. Gbb and Wit also signal non-canonically through the effector protein LIM kinase 1 (LIMK1) to regulate synapse stability. Interestingly, pMad also accumulates at synaptic locations, but the biological relevance of the phenomenon remained a mystery for over a decade. We found that synaptic pMad constitutes a sensor for synapse activity. Furthermore, we previously found that synaptic pMad marks a novel, noncanonical BMP pathway, genetically distinguishable from all other known BMP signaling cascades. This novel pathway stabilizes postsynaptic type-A (GluRIIA–containing) receptors as a function of their activity.
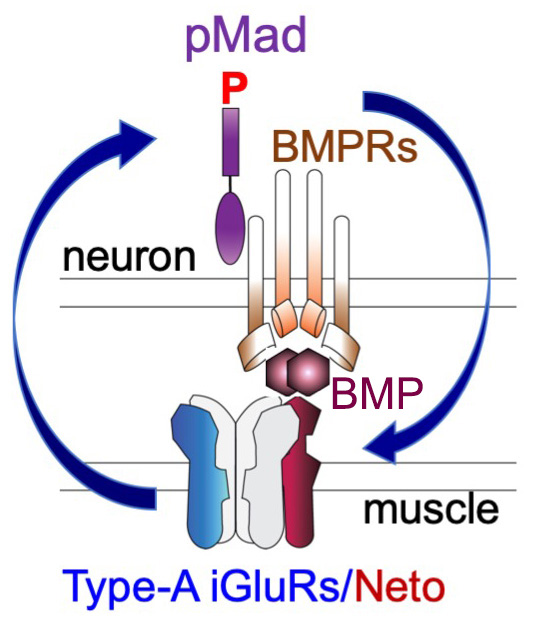
Type-A receptors are the first to arrive at a nascent synapse; they form the “core” of the receptor field and are surrounded by type-B receptors. Previous work in our and other labs established that the incorporation of type-A receptors in stable synaptic complexes depends on GluRIIA activity and requires an extensive postsynaptic network. Our studies on the novel BMP signaling modality indicate that the synaptic stabilization of type-A receptors also requires trans-synaptic complexes. The question arises as to how postsynaptic glutamate receptors modulate presynaptic pMad and are in turn stabilized by it. Given that synaptic pMad depends on active type-A receptors, we favor a model whereby Neto, via its BMP–binding CUB domains, connects active postsynaptic type-A receptors with presynaptic BMP/BMPR complexes (Figure 4). Such trans-synaptic complexes could offer a versatile means for relaying synapse activity status to the presynaptic neuron via fast conformational modifications. At the same time, the trans-synaptic complexes may facilitate interactions that stabilize the type-A receptors at PSDs, a positive feedback that could explain the Hebbian mode of GluRIIA incorporation at the PSD and maturation of iGluR fields at larval NMJs. Given that BMPRs are limiting and shared among different BMP signaling modalities, neurons may use this novel BMP pathway to monitor synapse activity and then coordinate NMJ growth with synapse maturation and stabilization.
Figure 4. The positive feedback loop model
Neto, via its BMP–binding CUB domains, connects active postsynaptic type-A channels with presynaptic BMP/BMP Receptor complexes. Active type-A channels trigger accumulation of presynaptic BMP/BMPR complexes (marked by pMad), which in turn stabilize the type-A channels at postsynaptic sites.
Selective disruption of synaptic BMP signaling by a Smad mutation adjacent to the conserved H2 helix
In search of Mad features that influence its association with the BMPRs, we collected most of existing Drosophila Mad alleles and compared them for their ability to sustain the two Smad–dependent signaling modalities: canonical BMP signaling, marked by pMad accumulation in motor neuron nuclei, and Smad–dependent local, synaptic signaling, marked by pMad accumulation at synaptic terminals. Within this comprehensive collection, we found that strong Mad alleles generally disrupt both synaptic and nuclear pMad accumulation, whereas moderate Mad alleles have a wider range of phenotypes and selectively impact different BMP signaling modalities. Interestingly, regulatory Mad mutations reveal that synaptic pMad appears to be more sensitive to a net reduction in Mad levels than is nuclear pMad. Importantly, a previously uncharacterized allele, Mad8 showed markedly reduced synaptic pMad levels but only moderately diminished nuclear pMad. The postsynaptic composition and electrophysiological properties of Mad8 NMJs were likewise altered. Using biochemical assays and structural modeling, we examined how point mutations such as S359L (marked*, Figure 5), present in Mad8, could influence the Mad- receptor interface, and we identified a key motif, the H2 helix [Reference 2]. Our study highlights the biological relevance of Smad–dependent, synaptic BMP signaling and uncovers a highly conserved structural feature of Smads that is critical for normal development and function.
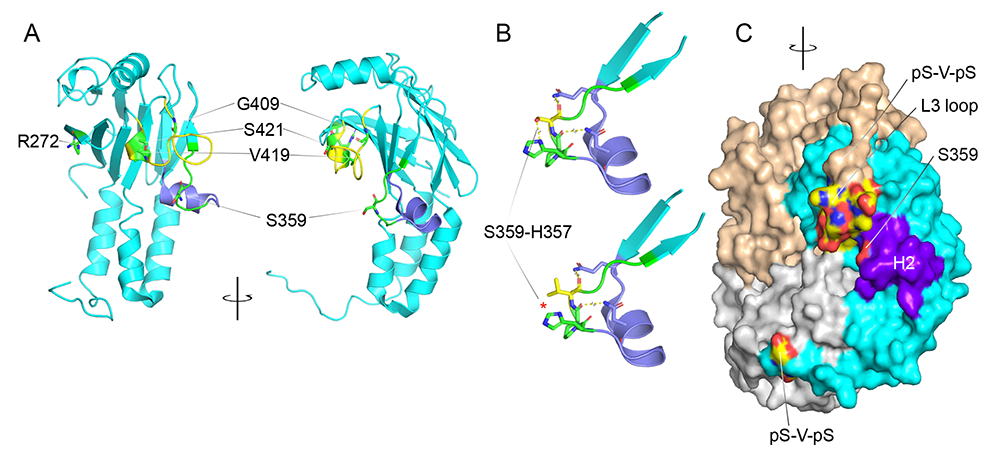
Figure 5. Structure of the MH2 domain of Drosophila Mad highlighting protein interaction motifs
A-C. Structure of the MH2 domain of Drosophila Mad (PDM code 3DIT) shown as monomer (A) and trimer (C). The two views of the structure (A) are related by a 90 rotation around a vertical axis. The L3 loop (yellow) is engaged in exclusive interactions with either the receptor or the phosphorylated C-tail of another MH2 domain. The H2 helix (purple) is critical for normal development and function, presumably because it mediates the interaction with the receptor. Residues mutated in various Mad alleles are mapped, including S359L, the Mad8 substitution.
A. Map of residues mutated in various Mad alleles, including S359L, the Mad8 substitution.
B. S359L in silico mutagenesis. S359 and its adjacent peptide backbone form hydrogen bonds with H357 and residues on the H2 helix (purple); the S359L substitution breaks the hydrogen bonds with H357 (red asterisk) and introduces a bulky moiety, shifting the H2.
C. Lateral view of the Mad MH2 trimer, showing the close proximity of the H2 helix to the charged L3 surface (colored by atoms).
Additional Funding
- NICHD Director’s Challenge Award Program in collaboration with Mary Dasso (NICHD) and Brian Oliver (NIDDK).
Publications
- Han TH, Vicidomini R, Ramos CI, Wang Q, Nguyen P, Jarnik M, Lee CH, Stawarski M, Hernandez RX, Macleod GT, Serpe M. Neto-alpha controls synapse organization and homeostasis at the Drosophila neuromuscular junction. Cell Rep 2020;32:107866.
- Nguyen TH, Han TH, Newfeld SJ, Serpe M. Selective disruption of synaptic BMP signaling by a Smad mutation adjacent to the highly conserved H2 helix. Genetics 2020;216:159-175.
- Arnaoutov A, Lee H, Plevock Haase K, Aksenova V, Jarnik M, Oliver B, Serpe M, Dasso M. IRBIT directs differentiation of intestinal stem cell progeny to maintain tissue homeostasis. iScience 2020;23(3):100954.
- Wang Q, Han TH, Nguyen P, Jarnik M, Serpe M. Tenectin recruits integrin to stabilize bouton architecture and regulate vesicle release at the Drosophila neuromuscular junction. eLife 2018;7:e35518.
Collaborators
- Mary Dasso, PhD, Section on Cell Cycle Regulation, NICHD, Bethesda, MD
- Gregory T. Macleod, PhD, Florida Atlantic University, Jupiter, FL
- Mark Mayer, PhD, Scientist Emeritus, NINDS, Bethesda, MD
- Stuart Newfeld, PhD, School of Life Sciences, Arizona State University, Tempe, AZ
Contact
For more information, email serpemih@mail.nih.gov or visit http://ucc.nichd.nih.gov.