Translational Biophotonics in Developmental Disorders and Diseases

- Amir H. Gandjbakhche, PhD, Head, Section on Biomedical Stochastic Physics
- Helga De Oliveira Ramirez, PhD, Postdoctoral Fellow
- Siddharth Khare, PhD, Postdoctoral Fellow
- Kosar Khaksari, PhD, Postdoctoral Fellow
- Thien Nguyen, PhD, Postdoctoral Fellow
- Soongho Park, PhD, Postdoctoral Fellow
- Hadis Dashtestani, MS, Intramural Research Training Award Student
- Wei Lun Huang, MS, Intramural Research Training Award Student
- Emily Blick, BS, Postbaccalaureate Fellow
- Kimberly Bress, BS, Postbaccalaureate Fellow
- Wisvanath Gorti, BS, Postbaccalaureate Fellow
- Ravi Malpani, BS, Postbaccalaureate Fellow
- John Millerhagen, BS, Postbaccalaureate Fellow
- Sheida Shahmohamadi, BS, Postbaccalaureate Fellow
- Marc Bornstein, PhD, Special Volunteer
- Han-Shin Hahn, PhD, Special Volunteer
- Siamak Aram, PhD, Guest Researcher
- Yasaman Ardeshirpour, PhD, Guest Researcher
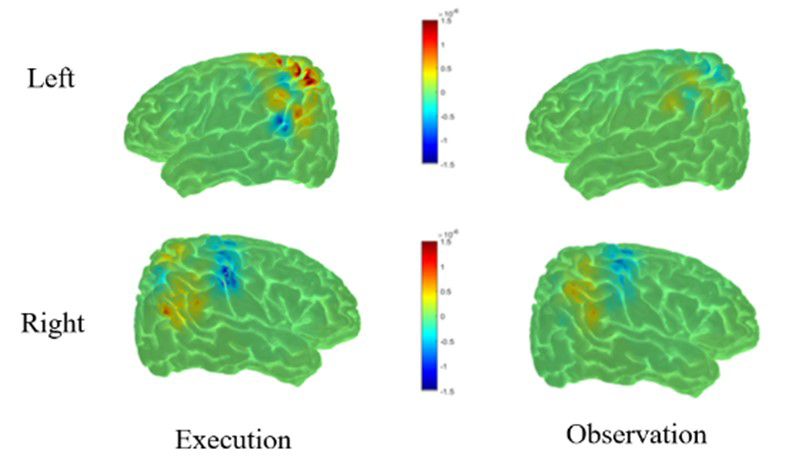
We continued to use near-infrared spectroscopy as a measure of brain activity to study developmental trajectories of cognitive abilities in children. We examined the feasibility of functional near infrared spectroscopy (fNIRS) for the study of the mirror neuron network (MNN, also referred to action-observation network [AON]) in a sample of 30 healthy controls who underwent a motor observation and execution paradigm while their brain activity was measured through EEG/fNIRS simultaneously. Overall, our results indicated that the parietal regions, including bilateral superior parietal lobule (SPL), bilateral inferior parietal lobule (IPL), right supra-marginal region (SMG) and right angular gyrus (AG) are candidate regions of the human AON (Figure 1).The AON is associated with the development of sophisticated social behaviors that emerge in typical human infants (e.g., complex imitation, decoding emotional states). Modeling MNN development using fNIRS (and EEG) will create a sensitive measure of deviations in social communication development before clinical behavioral deficits can be detected. To fully characterize the MNN using concurrent signals (EEG and fNIRS), we are currently conducting multimodal multiset data-fusion analysis with the goal of allowing the modalities to fully interact. After applying multiset canonical correlation analysis (mCCA) to the integrated datasets, we observed results consistent with previous literature. Preliminary results showed that action execution/observation exhibits a similar pattern of activity across regions of interest, indicating higher brain activity in regions in the left hemisphere (paracentral region, precentral region, and parietal inferior and superior regions) while subjects were performing an action. For the observation condition, higher brain activity was also found in left regions of the brain, namely the postcentral, paracentral, precentral, and parietal superior and inferior regions. This preliminary analysis is significant, because it is the first report describing the use of distinct brain metrics (hemodynamic response function and electrical activity) to characterize the MNN in the human brain. Future work includes testing the paradigm in a subset of typically developing infants and infants at risk for development/autism-spectrum disorder, who will be followed longitudinally. We will examine the developmental status of at-risk infants in relation to their initial neural data to determine whether MNN activation predicts developmental outcomes.
Figure 1.
HbO and HbR reconstruction maps for execution and observation in the left (top row) and right hemisphere (bottom row).
We finalized the project using fNIRS to examine the hemodynamic response in the prefrontal cortex (PFC) during a speech- and gesture-comprehension task [Reference 1]. Gesture abilities develop prior to speech, making gesture comprehension a detector of earlier aberrations in development. For this reason, we used both gesture and speech stimuli to examine how cortical activation may predict developmental differences. In children aged 18–36 months, we measured cortical activity by fNIRS while they were exposed to four types of stimuli: (1) meaningful gesture; (2) meaningless gesture; (3) meaningful speech; and (4) meaningless speech. Such stimuli allowed us to contrast brain activation across different types of communication and communicative intent. Children were also assessed at age three for language ability. The study showed differential activation to gesture compared with speech stimuli, as well as differential activation to meaningful versus non-meaningful stimuli. Importantly, the differences in mean activation in the left PFC in response to meaningful gesture (when controlling for meaningless gesture) at age two predicted verbal ability at age three. Differences in mean activation in response to meaningful speech compared with meaningful gesture at age two also predicted verbal ability at age three. The findings may reflect potential biomarkers for aspects of language development. Future research using larger samples of children with a wider array of language outcomes, such as children with specific language impairments, will be imperative for establishing whether this biomarker could be beneficial for early identification of aberrant language development.
We also used fNIRS to examine the underlying brain function in ornithine transcarbamylase deficiency (OTCD), the most common form of urea-cycle disorder, which is characterized by hyperammonia (HA). Using fNIRS, we examined the hemodynamics of the PFC in the OTCD population and in fraternal twins with and without OTCD. Results revealed a distinction in left PFC activation between controls and patients with OTCD, where controls showed higher task-related activation increase while performing the Stroop task. Subjects with OTCD also exhibited a bilateral increase in PFC activation. We quantified the hemodynamic variations in total hemoglobin, while twins performed the N-back working memory task [Reference 2].
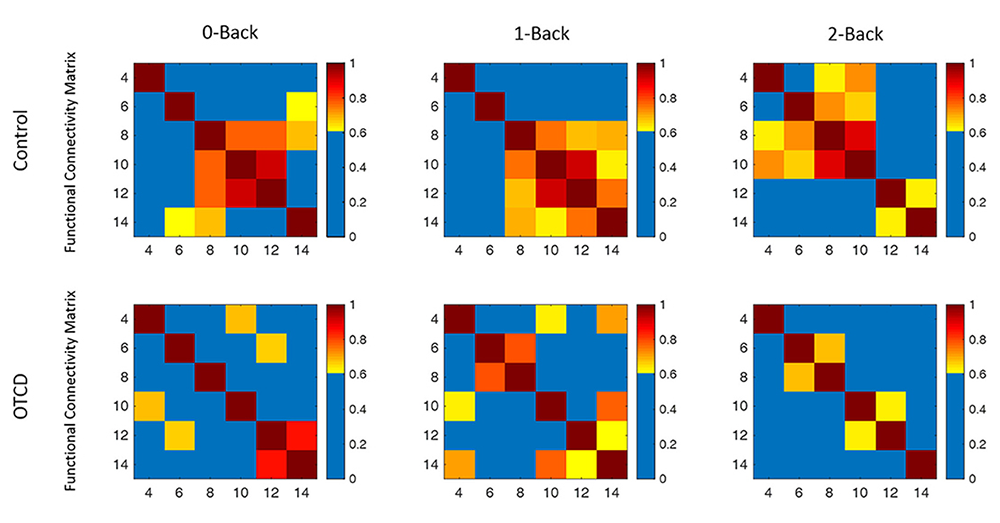
Our results showed that the sibling with OTCD had higher variations in a very low frequency band (less than 0.03 Hz, related to the mechanism of cerebral autoregulation) compared with the control sibling, possibly owing to the effect of HA. Functional connectivity (FC) analysis also revealed lower inter-hemispheric FC in an OTCD sibling as the task load increased (Figure 2). Our pilot results are the first to show the utility of fNIRS in monitoring OTCD cortical hemodynamics and in indicating the possibility of inefficient neurocognitive function [Reference 3].
Figure 2.
Functional connectivity map of the control and OTCD sibling during performance of working memory tasks
We are continuing to collaborate with Walter Reed Hospital on traumatic brain injury (TBI). Traumatic cerebral vascular injury (TCVI) is a frequent, but under-recognized, endophenotype of TBI. It likely contributes to functional deficits after TBI and TBI–related chronic disability, and represents an attractive target for targeted therapeutic interventions. The aim of this prospective study is to assess microvascular injury/dysfunction in chronic TBI by measuring cerebral vascular reactivity (CVR) by two methods: functional magnetic resonance imaging (fMRI) and fNIRS imaging, as each has useful features relevant to clinical utility. 42 subjects (27 chronic TBI, 15 age- and gender-matched non–TBI volunteers) were enrolled and underwent outpatient CVR testing by two methods: MRI-BOLD (BOLDscreen is an MRI–safe HD LCD monitor), and fNIRS, each with a hypercapnia challenge, a neuropsychological testing battery, and symptom-survey questionnaires. Chronic TBI subjects showed a significant reduction in global CVR compared with healthy controls. Our results show that pertinent parameters, such as mean CVR measured by fMRI and BOLD for non-TBI and TBI subjects, are different and correlate extremely well with CVR parameters obtained by fNIRS. Global CVR measured by fNIRS imaging also correlates with results obtained by MRI-BOLD. Focal CVR deficits seen on CVR maps by fMRI are also observed by fNIRS in the same areas in the frontal regions. Global CVR is significantly lower in chronic TBI patients and is reliably measured by both fMRI and fNIRS, the former with better spatial and the latter with better temporal resolution. Both methods show promise as non-invasive measures of CVR function and microvascular integrity after TBI [Reference 4].
We have begun to analyze data collected using fNIRS to validate cognitive tasks previously evaluated using fMRI. Using simultaneously collected fNIRS of the PFC and high-frequency heart-rate variability (HF-HRV), as derived from an electrocardiogram, we looked at the connection between prefrontal activation and parasympathetic nervous system activity (as measured HF-HRV) during a behavioral flexibility task (the go/no-go task).
COVID-19 point-of-care biosensor
The coronavirus disease 2019 (COVID-19) pandemic has created a challenge for researchers and healthcare professionals to design and test methods for screening and early detection of infected subjects, as well as for monitoring infected patients undergoing treatment. Using our expertise in tissue oxygenation, we are in the process of a clinical protocol approval to test a wearable multimodal biosensor. The device consists of a near-infrared spectroscopy (NIRS), a photoplethysmogram (PPG), and a thermometer sensor, capable of monitoring skin temperature, tissue oxygenation level, heart rate, and respiratory parameters. Data will be collected through a pilot study using this device in 40 healthy subjects who experienced a breathing pattern similar to that seen in pneumonia, using hypercapnia, paced breathing, and breath holding. We will identify vital parameters extracted from NIRS signals that could distinguish between normal and patterned breathing. The end product will be a point-of-care home-accessible device with Bluetooth functionality that is capable of identifying a COVID-19 infection. The study is a collaboration with Bruce Tromberg's Section and with Babak Shadgan. We are testing the first prototype of the wearable multimodal biosensor capable of monitoring skin temperature, tissue oxygenation, respiratory, and cardiac parameters.
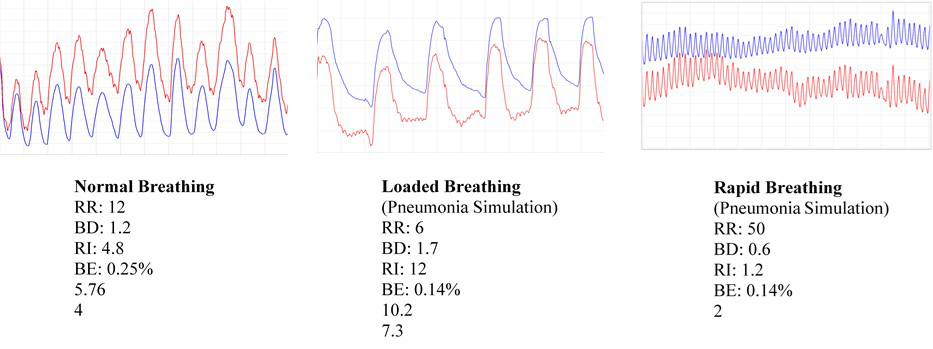
In the meantime, data collected by Babak Shadgan through a pilot study using this device in five healthy subjects, who experienced a breathing pattern simulating that seen in pneumonia, show encouraging results. We identified distinctive NIRS parameters that could distinguish between normal and loaded or shallow breathing. Respiratory rate (RR), respiratory rhythm (RT), breathing depth (BD), breathing interval (BI), breathing effort (BE) and inspiration slope (IS) are the respiratory-function parameters being considered in the first round of analysis; Figure 3 shows the distinctive patterns. In the next few months and as soon as the clinical protocol is approved, we will begin recruiting and will eventually apply our technology to COVID-19 patients on the NIH campus. Our ultimate goal is to use artificial intelligence and machine learning to identify a pattern of NIRS respiration and tissue oxygenation that would be specific to COVID-19.
Figure 3.
Respiratory function parameters for normal breathing vs. pneumonia simulation
Placenta oxygenation: from basics to point of care
Placental oxygenation plays a crucial role in a healthy pregnancy and its outcome. Defects in the placenta that affect placental oxygenation can cause preeclampsia, intrauterine growth restriction, fetal hypoxia, asphyxia, and cerebral palsy. A fast and non-invasive method that measures placental oxygenation quantitatively is necessary to detect such abnormalities. Current methods are either time consuming or not patient-friendly. We therefore developed a wearable device using NIRS that monitors anterior placenta oxygenation non-invasively and dynamically. The device uses two light sources, with 760 nm and 840 nm wavelengths, because they are sensitive to changes in blood oxyhemoglobin and deoxyhemoglobin. It consists of two photodiodes as detectors and six LED light sources, which are placed at six different distances, from 10 to 60 mm, from the LEDs. The different source and detector distances help us scan different tissue depths in order to distinguish between placental oxygenation and oxygenation of maternal layers. Also, the probe has a flexible geometry that enables us to place it in proper contact with the skin.
For the in vivo study, we tested the device on subjects in Detroit in collaboration with the Maternal-Fetal Medicine, Imaging, and Behavioral Development Affinity Group (Roberto Romero) at NICHD, Wayne State University, and with USUHS. The study focuses on baseline placental oxygenation for normal term pregnancies scheduled for cesarean section; ultra-sound imaging gives us the fat and uterus thicknesses that we need for the analysis. So far, we have measured placental oxygenation in 12 healthy, singleton, pregnant volunteers (33.3±3.6 weeks pregnant). We are in the process of completing our measurements on a total of 40 subjects to obtain adequate statistical power. The placental oxygenation calculated from two source-detector separations (30mm and 40mm) for this group of 12 subjects ranges from 68% to 89%. However, we found that the calculated placental oxygenation is positively correlated with the thickness of the fat layer: pregnant women with a thicker fat layer display higher placental oxygenation. We believe that the correlation was caused by the highly scattering characteristic of the fat.
We are thus now performing a Monte Carlo simulation on a five-layer model to correct for the effect of maternal layers such as fat on placental oxygenation. The simulations are based on thickness and on both the scattering and absorption coefficients of all maternal layers (dermis, epidermis, fast, uterus) and placenta. Although the placenta is an essential organ for fetal development and successful reproduction, of these it is the least-studied organ. We therefore also measured the scattering coefficients of the human placenta in the range of 659 to 840nm, using a well-established frequency domain diffuse optical spectroscopic system (DOSI) and a lab-designed diffuse reflectance device (DRS). Measurements were performed on eight placentas obtained after cesarean deliveries. We then calculated absorption and scattering coefficients calculated from the measured reflectance using the random-walk theory for DRS and a frequency-domain algorithm for DOSI. Average reduced scattering coefficient (µs′) was 0.943 ± 0.015 mm-1 at 760 nm and 0.831 ± 0.009 mm-1 at 840 nm. A power law with an exponent of 1.426 describes accurately changes of human placental scattering coefficient as a function of wavelength. Such scattering coefficients can be used to improve measurements of placental oxygen saturation [Reference 5].
Along with the in vivo studies, we are studying placental oxygenation at the cellular level using a novel biophotonics method named dynamic full-field optical coherence tomography (DFFOCT) and in HeLa cells with manually changed oxygenation. The preliminary results established the ability of DFFOCT to detect the changes in intracellular activity for different oxygen levels. HeLa cell samples were treated with Triton X-100, which causes membrane permeabilization, and paraformaldehyde, which causes cell fixation. We imaged untreated and treated samples using DFFOCT to determine whether it could detect cellular activity. We were able to isolate cellular signals from the environment and measure changes in cellular activity following various inhibition treatments, highlighting the potential of DFFOCT to uncover new information about dynamic intracellular fluctuations during various cellular processes. Future experiments with targeted cellular treatments can be conducted to further characterize cellular activity. To identify the biological causes of the signal from untreated samples, we plan controlled experiments involving the suppression of cellular energetics by mitochondrial inhibitors and glucose decouplers. Cellular energetics are essential for large polymer buildup, disassembly, movement within the cell, and small protein activity.
Tissue characterization and function
We are investigating photonic techniques to elucidate biomarkers for the diagnosis of diseases or the assessment of treatment outcome across a variety of conditions. We are assessing facial plethora in Cushing’s syndrome (CS), as it was one of the earliest described clinical features of the disease. In collaboration with Constantine Stratakis, we quantified changes in facial plethora in CS as an early assessment of cure. We performed noninvasive multispectral NIR imaging on the right cheek of patients before and after surgery. Patients were defined as cured by postoperative levels of plasma cortisol of less than 3 mcg/dl and/or by adrenocortical insufficiency, for which they received replacement therapy. Results indicate that a reduction in facial plethora after surgery, as evidenced by a reduction in blood volume fraction, is correlated with the cure of CS. The first set of results were published in 2015. In our follow-up paper [Reference 6], we also showed that the water-content fraction can be used as a new biomarker of early cure in patients with CS. We recorded data for 29 new patients, and follow-up imaging was conducted for 26 patients. We also developed and tested a new hand-held system that has improved performance over the existing portable system. We plan to use the system as a point-of-care imaging device. In brief, the new imager uses a high-resolution complementary metal oxide semiconductor (CMOS) camera with on-chip filters. Images are acquired simultaneously at eight different near-infrared wavelengths (700–980 nm). Our graphical user interface (Figure 3c) now supports both portable and the hand-held multispectral imagers.
Annually, about 15 million preterm infants are born in the world. Of these, about 1 million die before the age of five because of complications resulting from their premature birth. Given that the high incidence of preterm birth (PTB) is partially the result of the lack of effective diagnostic modalities, methodologies are needed to determine the risk of PTB. We proposed a non-invasive tool based on polarized-light imaging aimed at measuring the organization of collagen in the cervix. Cervical collagen has been shown to remodel with the approach of parturition. We used a full-field Mueller matrix polarimetric colposcope to assess and compare cervical collagen content and structure in nonpregnant and pregnant women. The local collagen directional azimuth was used, and we imaged a total of eight cervices. In a continued collaboration with Jessica Ramella-Roman on preterm pregnancy complications, we used the Preterm Imaging system based on colposcopy to characterize uterine cervix structure in a longitudinal study of low-risk and high-risk (i.e., prior PTB or a sonographic short cervix) patients. Polarization imaging is an effective tool to measure optical anisotropy in birefringent materials, such as the cervix's extracellular matrix, and to predict cervical ripening. For this reason, it has potential to predict preterm birth. Through our collaborations with Roberto Romero’s Branch and with Jessica Ramella-Roman, we will test the system in a control population and those with PTB prevalence [Reference 5].
Additional Funding
- Bench to Bedside Award 345 (2016): “Mirror neuron network dysfunction as an early biomarker of neurodevelopment” (ongoing)
- Human Placenta Project–NICHD (2016, ongoing)
Publications
- Smith EG, Condy E, Anderson AA, Thurm A, Manwaring SS, Swineford L, Gandjbakhche AH, Redcay E. Functional near-infrared spectroscopy in toddlers: neural differentiation of communicative cues and relation to future language abilities. Dev Sci 2020;23:e12948.
- Anderson AA, Gropman A, Le Mons C, Stratakis C, Gandjbakhche AH. Evaluation of neurocognitive function of prefrontal cortex in ornithine transcarbamylase deficiency. Mol Genet Metab 2020;129:207-212.
- Anderson AA, Gropman A, Le Mons C, Stratakis CA, Gandjbakhche AH. Hemodynamics of prefrontal cortex in ornithine transcarbamylase deficiency: a twin case study. Front Neurol 2020;11:809-812.
- Amyot F, Kenney K, Spessert E, Moore C, Haber M, Silverman E, Gandjbakhche A, Diaz-Arrastia R. Assessment of cerebrovascular dysfunction after traumatic brain injury with fMRI and fNIRS. Neuroimage Clin 2020;25:102086.
- Khare S, Nguyen T, Anderson AA, Hill B, Romero R, Gandjbakhche AH. Evaluation of the human placenta optical scattering properties using continuous wave and frequency-domain diffuse reflectance spectroscopy. J Biomed Optics 2020;25:116001.
- Afshari A, Keil M, Lyssikatos C, Belyavskaya E, Valdés N, Chowdhry FA, Parsa K, Ardeshirpour Y, Pursley R, Khare S, Kainerstorfer JM, Chittiboina P, Lodish MB, Mazzuchi TA, Gandjbakhche AH, Stratakis CA. Optical imaging technology: a useful tool to identify remission in Cushing disease after surgery. Horm Metab Res 2019;51(2):120-126.
Collaborators
- Franck Amyot, PhD, Center for Neuroscience and Regenerative Medicine, Uniformed Services University of the Health Sciences (USUHS), Bethesda, MD
- Mehran Armand, PhD, The Johns Hopkins University, Baltimore, MD
- Claude Boccara, PhD, École Supérieure de Physique et de Chimie Industrielles, Paris, France
- Andrea Gropman, MD, Children's National Health System, Washington, DC
- Sonia S. Hassan, MD, Wayne State University School of Medicine, Detroit, MI
- Tom Pohida, MS, Division of Computational Bioscience, CIT, NIH, Bethesda, MD
- Jay Knutson, PhD, Laboratory of Molecular Biophysics, NHLBI, Bethesda, MD
- Randall Pursley, Signal Processing and Instrumentation Section, CIT, NIH, Bethesda, MD
- Jessica C. Ramella-Roman, PhD, Florida International University, Miami, FL
- Roberto Romero-Galue, MD, Perinatology Research Branch, NICHD, Detroit, MI
- Dan Sackett, PhD, Division of Basic and Translational Biophysics, NICHD, Bethesda, MD
- Babak Shadgan, MD, MSc, PhD, University of British Columbia, Vancouver, Canada
- Constantine Stratakis, MD, D(med)Sci, Section on Endocrinology and Genetics, NICHD, Bethesda, MD
- Audrey Thurm, PhD, Pediatrics & Developmental Neuropsychiatry Branch, NIMH, Bethesda, MD
- Bruce Tromberg, PhD, Section on Biomedical Optics, NICHD, Bethesda, MD
- Eric Wassermann, MD, Cognitive Neuroscience Section, NINDS, Bethesda, MD
Contact
For more information, email gandjbaa@mail.nih.gov or visit https://irp.nih.gov/pi/amir-gandjbakhche.