Thyroid Hormone Regulation of Vertebrate Postembryonic Development

- Yun-Bo Shi, PhD, Head, Section on Molecular Morphogenesis
- Liezhen Fu, PhD, Staff Scientist
- Nga Luu, MS, Biologist
- Lusha Liu, PhD, Visiting Professor
- Lu Xue, PhD, Visiting Professor
- Wonho Na, PhD, Visiting Fellow
- Yuki Shibata, PhD, Visiting Fellow
- Yuta Tanizaki, PhD, Visiting Fellow
- Lingyu Bao, MS, Graduate Student
- Shouhong Wang, BS, Graduate Student
The laboratory investigates the molecular mechanisms of thyroid hormone (TH) function during postembryonic development, a period around mammalian birth when plasma TH levels peak. The main model is the metamorphosis of Xenopus laevis and X. tropicalis, two highly related species, which offer unique but complementary advantages. The control of this developmental process by TH offers a paradigm in which to study gene function in postembryonic organ development. During metamorphosis, different organs undergo vastly different changes. Some, like the tail, undergo complete resorption, while others, such as the limb, are developed de novo. The majority of larval organs persist through metamorphosis but are dramatically remodeled to function in a frog. For example, tadpole intestine is a simple tubular structure consisting primarily of a single layer of larval epithelial cells. During metamorphosis, it is transformed, through specific larval epithelial cell death and de novo development of the adult epithelial stem cells, followed by their proliferation and differentiation, into an organ with a multiply folded adult epithelium surrounded by elaborate connective tissue and muscles. The wealth of knowledge from past research and the ability to manipulate amphibian metamorphosis, both in vivo by using genetic approaches or hormone treatment of whole animals, and in vitro in organ cultures, offer an excellent opportunity firstly to study the developmental function of TH receptors (TRs) and the underlying mechanisms in vivo and, secondly, to identify and functionally characterize genes that are critical for organogenesis, particularly for the formation of the adult intestinal epithelial stem cells during postembryonic development in vertebrates. A major recent focus has been to make use of the TALEN and CRISPR/Cas9 technologies to knockdown or knockout the endogenous genes for functional analyses. In addition, the recent improvements in Xenopus tropicalis genome annotation allow us to carry out RNA-Seq and chromatin-immunoprecipitation (ChIP)-Seq analyses at the genome-wide level. We complement our frog studies by investigating the genes found to be important for frog intestinal stem-cell development in developing mouse intestine by making use of the ability to carry out conditional gene knockout.
Identification of notochord-enriched genes induced during Xenopus tropicalis tail resorption
Tail resorption during anuran metamorphosis is perhaps the most dramatic tissue transformation to occur during vertebrate development. Like all other processes during metamorphosis, tail resorption is controlled by TH. Earlier studies in the highly related anuran species Xenopus laevis and Xenopus tropicalis showed that TR plays a necessary and essential role in metamorphosis. Of the two known TR genes in all vertebrates, trα is highly expressed during both premetamorphosis and metamorphosis, while trβ expression is low in premetamorphic tadpoles but highly upregulated as a direct target gene of TH during metamorphosis, especially in the tail. Gene knockout studies showed that trβ knockout significantly delays late metamorphosis, particularly tail resorption, resulting in tailed frogs well after wild-type siblings complete metamorphosis. Most noticeably, in trβ-knockout tadpoles, an apparently normal notochord is present in the tail as late as three days after the initiation of tail shortening (stage 62), while in wild-type and trα-knockout tadpoles, the tail notochord disappears in about one day. The findings suggest the existence of TRβ-regulated notochord-specific gene expression program during tail resorption.
We carried out a comprehensive gene expression analysis in the notochord during metamorphosis using RNA-Seq analyses of whole tail at stage 60 before any noticeable tail length reduction, whole tail at stage 63 when the tail length is reduced by about one half, and the rest of the tail at stage 63 after removing the notochord. This allowed us to identify many notochord-enriched, metamorphosis-induced genes at stage 63 [Reference1]. Future studies on these genes should help determine whether they are regulated by TRb and play any roles in notochord regression. We also discovered differential regulation of several matrix metalloproteinases (MMPs), which are known to be upregulated by TH and thought to play a role in tissue resorption by degrading the extracellular matrix (ECM). In particular, MMP9-TH and MMP13 are extremely highly expressed in the notochord compared with the rest of the tail. In situ hybridization analyses showed that these MMPs are expressed in the outer sheath cells and/or the connective tissue sheath surrounding the notochord. Our findings suggest that high levels of trβ expression in the notochord specifically upregulate the MMPs, which in turn degrades the ECM, leading to the collapse of the notochord and its subsequent resorption during metamorphosis.
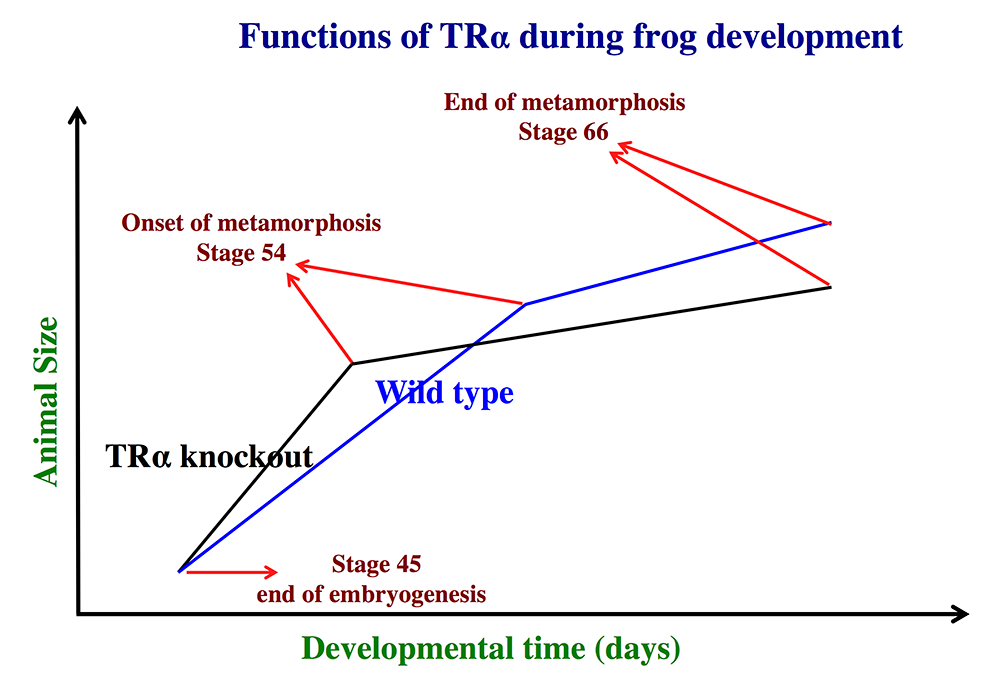
Figure 1. Schematics showing the effects of TRα (thyroid hormone receptor α) knockout on Xenopus tropicalis development
TRα knockout has little effect on embryogenesis, and resulting tadpoles are normal by feeding stage (stage 45). However, once feeding begins, the animals grow at different rates, with the knockouts growing faster; they are thus larger than wild-type siblings at the same age (in days) (comparing the vertical axis values of the lines for the knockout and wild-type animals at any given position along the horizontal axis between stages 45 and 54). The knockout animals also develop faster, reaching developmentally more advanced stages than wild-type siblings at the same age (in days). Thus, the knockout animals reach stage 54, the onset of metamorphosis, at a younger age (see the horizontal axis locations for the upper end of the lines). Interestingly, when the animals are compared at stage 54, the wild-type are larger than the knockout siblings, even though the latter grow faster. This is because the wild-type animals take longer to reach metamorphosis (stage 54). The extra growth time needed to reach stage 54 enables the wild-types to catch up and surpass the knockouts in size. After the initiation of metamorphosis at stage 54, the knockout tadpoles metamorphose more slowly than the wild-types, enabling the latter to catch up in development, with both groups and finishing metamorphosis at around the same age. The knockout animals initiate metamorphosis at a smaller size and also end up smaller at the end of metamorphosis than do the wild-type siblings. Thus, in premetamorphic tadpoles prior to stage 54, unliganded TRα (due to the lack of thyroid hormone) functions to control metamorphic timing, whereas, when thyroid hormone becomes available during metamorphosis, TRα helps increase the rate of metamorphosis.
Organ-specific requirements for thyroid hormone receptor ensure temporal coordination of tissue-specific transformations and completion of Xenopus metamorphosis.
We generated trα and trβ double knockout animals and carried out molecular and morphological analyses to determine whether TR is required for Xenopus development [Reference 2]. We found that the tr double knockout tadpoles do not respond to TH, supporting the view that there are no other TR genes in Xenopus tropicalis and that TR is essential for mediating the effects of TH in vivo. Surprisingly, the double knockout tadpoles are able to initiate metamorphosis and accomplish many metamorphic changes, such as limb development. However, all double knockout tadpoles stall and eventually die at stage 61, the climax of metamorphosis, before tail resorption takes place. Analyses of the knockout tadpoles at stage 61 revealed various developmental abnormalities, including precocious ossification and extra vertebrae. Our data indicate that TRs are not required for the initiation of metamorphosis but are essential for its completion. Furthermore, the differential effects of tr knockout on different organs/tissues suggest tissue-specific roles for TR to control temporal coordination and progression of metamorphosis in various organs.
Function of the TR-coactivator histone methyltransferase PRMT1 during Xenopus tropicalis development
Asymmetric arginine dimethylation of histone H4R3 to H4R3me2a by protein arginine methyltransferase 1 (PRMT1), a TR-coactivator that is also upregulated by TH during metamorphosis, is thought to play a key role in gene activation throughout vertebrates. PRMT1 knockout in mouse leads to embryonic lethality. This and the uterus-enclosed nature of the mouse embryo make it difficult to determine the role of PRMT1 in mammal development. We took advantage of the external development of the diploid anuran Xenopus tropicalis and adapted the TALEN genome editing technology to knock out PRMT1 in order to investigate how PRMT1 participates in vertebrate development [Reference 3]. We observed that PRMT1 knockout had no apparent effect on embryogenesis because normally feeding tadpoles were formed, despite the reduced asymmetric H4R3 di-methylation (H4R3me2a) resulting from the knockout. However, PRMT1 knockout tadpoles exhibited severely reduced growth even with normal growth-hormone gene expression. The development of such tadpoles was also stalled shortly after feeding began at stages 44/45, and they died within two weeks, well before the onset of metamorphosis. In situ analyses revealed broad cessation of or drastic reduction in cell proliferation in diverse organs including the eye, brain, spinal cord, liver, and intestine. Our findings suggest that PRMT1 is not required for embryogenesis but is a key regulator in the normal progression of vertebrate development and growth.
TRb is critical for intestinal remodeling during Xenopus tropicalis metamorphosis.
We analyzed the effect of trβ knockout on TH–induced intestinal remodeling using animals containing an out-frame-mutation of a five-base deletion generated with the CRISPR/Cas9 gene-editing technology [Reference 4]. We observed that trβ knockout does not affect premetamorphic tadpole development. However, we found that the basal expression of direct TH–inducible genes is increased and their upregulation by TH is reduced in the intestine of premetamorphic homozygous trβ knockout animals and is accompanied by reduced target binding by TR. More importantly, we observed reduced adult stem cell proliferation and larval epithelial apoptosis in the intestine during TH–induced metamorphosis. Our data suggest that TRβ plays a critical role in intestinal remodeling during metamorphosis.
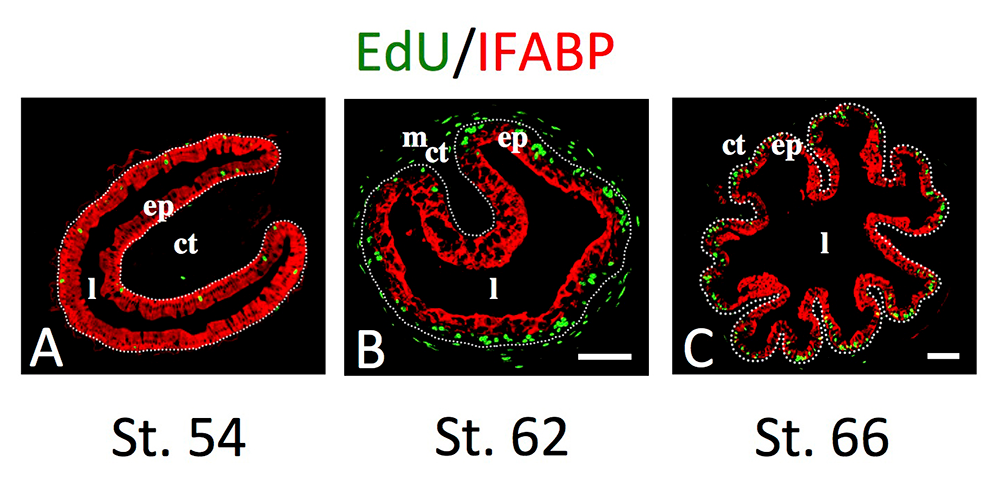
Figure 2. Intestinal metamorphosis involves the formation of clusters of proliferating, undifferentiated epithelial cells at the climax.
Tadpoles at premetamorphic stage 54 (A), climax (B, stage 62), and end of metamorphosis (C, stage 66) were injected with 5-ethynyl-2′-deoxyuridine (EdU) one hour before being sacrificed. Cross-sections of the intestine from the resulting tadpoles were double-stained by EdU labeling of newly synthesized DNA and by immunohistochemistry of IFABP (intestinal fatty acid–binding protein), a marker for differentiated epithelial cells. The dotted lines depict the epithelium-mesenchyme boundary. Note that there are few EdU–labeled proliferating cells in the epithelium and that they express IFABP at premetamorphosis (A) and increase in the form of clustered cells (proliferating adult stem cells), which lack IFABP at the climax of metamorphosis (B). At the end of metamorphosis, EdU–labeled proliferating cells are localized mainly in the troughs of the epithelial folds, where IFABP expression is low (C). ep, epithelium; ct, connective tissue; m, muscles; l, lumen.
The tRNA methyltransferase-like 1 gene (Mettl1) is directly regulated by thyroid hormone receptor during Xenopus tropicalis metamorphosis, implicating a role in adult intestinal stem-cell development and proliferation.
We previously used ChIP-on-chip assays to identify many putative TR target genes, among them the tRNA methyltransferase Mettl1. We studied the regulation of the mettl1 gene by TH during intestinal metamorphosis, a process that involves near complete degeneration of the larval epithelial cells via apoptosis and de novo formation of adult epithelial stem cells and their subsequent proliferation and differentiation. We observed that mettl1 was activated by TH in the intestine during both natural and TH–induced metamorphosis and that its mRNA level peaks at the climax of intestinal remodeling. We further showed that the mettl1 promoter could be activated by liganded TR via a TH response element (TRE) upstream of the transcription start site in vivo. More importantly, we found that TR binding to the TRE region correlated with the increase in the level of H3K79 methylation, a transcription-activation histone mark, and the recruitment of RNA polymerase II by TH during metamorphosis. Our findings suggest that, in the intestine during metamorphosis, mettl1 is activated by liganded TR directly at the transcriptional level via the TRE in the promoter region. Mettl1 in turn regulates target tRNAs to affect translation, thus facilitating stem-cell formation and/or proliferation during intestinal remodeling.
Wnt promotes amino-acid transporter LAT1 to constrain the integrated stress response during mouse embryogenesis.
To regulate cellular processes, TH has to be actively transported into cells, a process that is mediated by several different types of transporters. One of our previously identified TH–response genes in the intestine, lat1, encodes the light chain of a heterodimeric system L type of TH transporter, which also transports several amino acids. Interestingly, lat1 is highly upregulated at the climax of metamorphosis in the tadpole intestine, coinciding with the formation and rapid proliferation of the adult intestinal stem cells. In addition, we found that Lat1 was also highly expressed in the mouse intestine during the neonatal period when the mouse intestine matures into the adult form, a process that appears also involves TH–dependent formation and/or proliferation of the adult intestinal stem cells. Through collaborative studies, we generated a mouse line with the Lat1 gene floxed, which allows conditional knockout of the Lat1v upon expression of the Cre recombinase. More recently, we showed that Lat1 is highly expressed in mouse tissues undergoing morphogenesis and that Lat1–null mouse embryos, generated by crossing the Lat1–floxed mice with a mouse line expressing Cre ubiquitously, have profound neural and limb-bud outgrowth defects [Reference 5]. Lat1–null neural tissue exhibited mild proliferation defects and aberrant mTORC1 activity; transcriptomics and protein phosphorylation and apoptosis analyses further indicated that induction of the integrated stress response is the likely cause of the observed defects. We also detected the pattern of stress-response gene expression induced in Lat1–null embryos at a low-level in wild-type embryos and identified stress-vulnerability specifically in tissues undergoing morphogenesis. The Lat1–null phenotype is reminiscent of Wnt–pathway mutants, and we showed that loss of Wnt or β-catenin inhibits Lat1 expression and induces the stress response. Wnt signaling therefore normally supports the metabolic demands of morphogenesis and constrains cellular stress. Moreover, operation of the integrated stress response in the embryo, which is pathogen-mediated as well as triggered by metabolic stress, may provide a mechanistic explanation for a range of developmental defects.
Additional Funding
- Japan Society for the Promotion of Science (JSPS) fellowships for Drs. Yuta Tanizaki and Yuki Shibata
- Awards from the China Scholarship Council (CSC) to Drs. Shouhong Wang, Lu Xue, and Lusha Liu
Publications
- Nakajima K, Tanizaki Y, Luu N, Zhang H, Shi Y-B. Comprehensive RNA-Seq analysis of notochord-specific genes induced during Xenopus tropicalis tail resorption. Gen Comp Endocrinol 2020;287:113349.
- Shibata Y, Wen L, Okada M, Shi Y-B. Organ-specific requirements for thyroid hormone receptor ensure temporal coordination of tissue-specific transformations and completion of Xenopus metamorphosis. Thyroid 2020;30:300–313.
- Shibata Y, Okada M, Miller TC, Shi Y-B. Knocking out histone methyltransferase PRMT1 leads to stalled tadpole development and lethality in Xenopus tropicalis. BBA-Gen Subjects 2020;1864:129482.
- Shibata Y, Tanizaki Y, Shi Y-B. Thyroid hormone receptor beta is critical for intestinal remodeling during Xenopus tropicalis metamorphosis. Cell Biosci 2020;10:46.
- Poncet N, Gierlinski M, Melanie Febrer M, Lipina C, Halley PA, Shi Y-B, Yamaguchi TP, Taylor PM, Storey KG. Wnt promotes amino acid transporter Slc7a5 to constrain the integrated stress response during mouse embryogenesis. EMBO Rep 2020;21:e48469.
Collaborators
- Sheue-Yann Cheng, PhD, Laboratory of Molecular Biology, NCI, Bethesda, MD
- Steven Coon, PhD, Molecular Genomics Core, NICHD, Bethesda, MD
- Eiichi Hinoi, PhD, Kanazawa University Graduate School, Kanazawa, Japan
- James Iben, PhD, Molecular Genomics Core, NICHD, Bethesda, MD
- Jianping Jiang, PhD, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, China
- Tianwei Li, PhD, Molecular Genomics Core, NICHD, Bethesda, MD
- Keisuke Nakajima, PhD, Amphibian Research Center, Hiroshima University, Hiroshima, Japan
- Bingyin Shi, MD, Xi’an Jiaotong University School of Medicine, Xi'an, China
- Kate G. Storey, PhD, University of Dundee, Dundee, UK
- Guihong Sun, PhD, Wuhan University School of Medicine, Wuhan, China
- Peter Taylor, PhD, University of Dundee, Dundee, UK
- Henry Zhang, PhD, Bioinformatics and Scientific Programming Core, NICHD, Bethesda, MD
Contact
For more information, email shi@helix.nih.gov or visit http://smm.nichd.nih.gov.