Organ and Tissue Formation during Development

- Brant M. Weinstein, PhD, Head, Section on Vertebrate Organogenesis
- Aniket Gore, PhD, Staff Scientist
- Van Pham, BS, Scientific Technician
- Leah Greenspan, PhD, Postdoctoral Fellow
- Hyun Min Jung, PhD, Postdoctoral Fellow
- Miranda Marvel, PhD, Postdoctoral Fellow
- Scott Paulissen, PhD, Postdoctoral Fellow
- Laura Pillay, PhD, Postdoctoral Fellow
- Kiyohito Taimatsu, PhD, Postdoctoral Fellow
- Marina Venero Galanternik, PhD, Postdoctoral Fellow
- Olivia Carpinello, MD, Medical Fellow
- Maziar Rahmani, MD, Medical Fellow
- Alexandra Fister, BS, Postbaccalaureate Fellow
- Bakary Samasa, BS, Postbaccalaureate Fellow
- Avery Swearer, BS, Postbaccalaureate Fellow
- Joseph Yano, BS, Postbaccalaureate Fellow
The major focus of the Section is to understand how the elaborate networks of blood and lymphatic vessels arise during vertebrate development. Blood vessels supply every tissue and organ with oxygen, nutrients, and cellular and humoral factors. Lymphatic vessels drain fluids and macromolecules from the interstitial spaces of tissues, returning them to the blood circulation, and they play an important role in immune responses. Our studies on the formation of blood and lymphatic vessels are of great clinical interest because of the roles both types of vessels play in cancer and ischemia.
The zebrafish (Danio rerio), a small tropical freshwater fish, possesses a unique combination of features that make it particularly suitable for studying vessel formation. Zebrafish are genetically tractable vertebrates with externally developing, optically clear embryos, which are readily accessible for observation and experimental manipulation. Such features permit observation of every vessel in the living animal and simple, rapid screening for even subtle vascular-specific defects. Our current studies use genetic screening, experimental analysis, and imaging to examine cues directing vascular patterning and morphogenesis, regulation of vascular integrity, assembly of the lymphatic system, and the roles of novel vascular-associated cells.
In addition to our work on vessel development, we are pursuing studies on the role of epigenetics during early development, in particular how DNA methylation and other epigenetic mechanisms help coordinate cell, tissue, and organ specification and differentiation.
Specification and patterning of developing blood vessels
We are working to elucidate the cellular and molecular mechanisms responsible for the specification, patterning, and differentiation of blood vessels during development. Blood vessels are ubiquitous and vital components of vertebrate animals, innervating and supplying every tissue and organ with oxygen and nutrients. Many of the recent insights into mechanisms of blood vessel formation have come from studies in model organisms including the zebrafish. In zebrafish every blood vessel can be observed in living animals with high resolution, and simple, rapid screening can be accomplished for even subtle vascular-specific mutants (Figure 1). We are carrying out several related projects using the fish, which are described below.
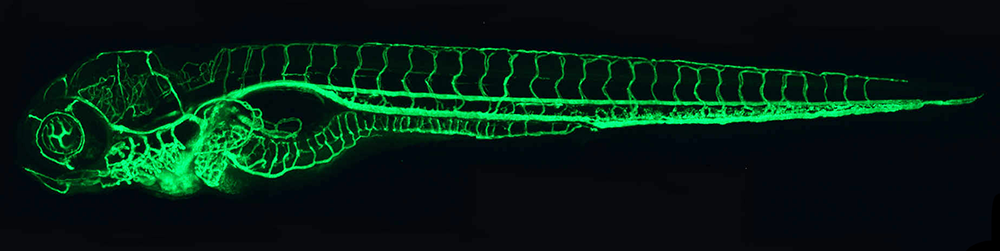
Figure 1. The zebrafish vascular system
Confocal micro-angiogram of the vascular system of a 4½-day-old zebrafish larva labeled by injecting fluorescent microspheres. The transparency of zebrafish larvae makes it possible to use high-resolution optical imaging methods to visualize the entire vasculature in exquisite detail.
New tools for experimental analysis of vascular development
We generate novel transgenic lines for visualizing different endothelial cell and perivascular cell types and for driving gene expression or performing molecular profiling of mRNAs and microRNAs in these cell populations.
Genetic analysis of vascular development
We have identified many novel mutants affecting vascular development in our transgene-assisted forward-genetic screens and are currently characterizing the phenotypes and molecular basis for several of such mutants.
Analysis of vascular specification, patterning, and morphogenesis
We are studying the development of several vascular beds, including the vasculature of the pectoral fin, the fish equivalent of the mammalian forelimb.
Regulation of vascular integrity
We are using the zebrafish to understand the cellular and molecular mechanisms responsible for proper vessel morphogenesis and for the generation and maintenance of vascular integrity. Disruption of vascular integrity is associated with hemorrhagic stroke, a severe and debilitating form of stroke associated with high morbidity and mortality. Meningeal vascular dysfunction is also associated with neurocognitive deficits and neurodegenerative disease. Many of the recent insights into the molecular mechanisms regulating vascular integrity have come from studies in model organisms such as the zebrafish. We are pursuing several related projects.
Genes regulating vascular integrity
We used forward-genetic screens to identify new zebrafish mutants that disrupt cranial vascular integrity in the zebrafish (Figure 2), using next-gen sequencing methods to accomplish higher throughput cloning of mutants. We already characterized the role of GDF6 (growth differentiation factor 6, also known as BMP13) in vascular integrity, demonstrating that the gene promotes maintenance of vascular integrity by suppressing excess VEGF (vascular endothelial growth factor) signaling. We are currently characterizing the molecular nature of defects in the regulatory protein RHOA (involved in cytoskeletal dynamics, transcription, cell cycle progression, and cell transformation), which result in vascular integrity defects.
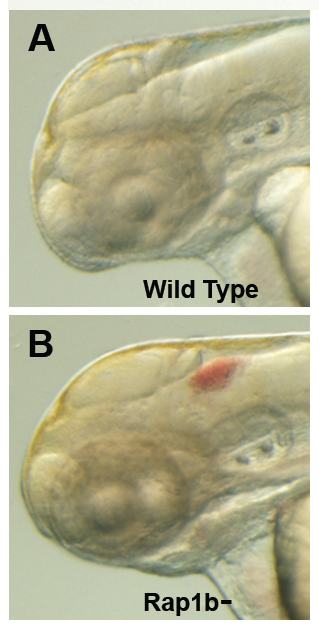
Figure 2. Intracranial hemorrhage (ICH) in the developing zebrafish
The clarity of zebrafish larvae also makes it straightforward to screen for animals with intracranial hemorrhage, as is evident in comparing lateral views of a 2-day-old wild-type larva (A) with a hemorrhage-prone larva deficient in rap1b (B).
Acquisition and function of supporting vascular smooth muscle cells
The vascular smooth cells (VSMC) that surround the endothelial tube play a critical role in regulating vascular tone and vascular integrity. We examined the early origins of the cells, how their interaction with endothelial tubes helps maintain the vascular basement membrane and restricts vessel diameter, and the molecular mechanisms underlying the arterial (versus venous) specific recruitment of VSMC.
Vasculature and vascular-associated cells in the meninges
The meninges are an external enveloping connective tissue that encases the brain, producing cerebrospinal fluid, acting as a cushion against trauma, nourishing the brain via nutrient circulation, and removing waste. Despite its importance, the cell types present in the meninges and the function and embryonic origins of the tissue are still not well understood. We recently discovered and characterized fluorescent granular perithelial cells (FGPs) in the zebrafish, a novel endothelium-derived perivascular cell population closely associated with meningeal blood vessels, which is likely to play a critical role in meningeal function (Figure 3). We are currently carrying out additional studies to understand the function of FGPs and other novel meningeal vascular-associated cell populations.
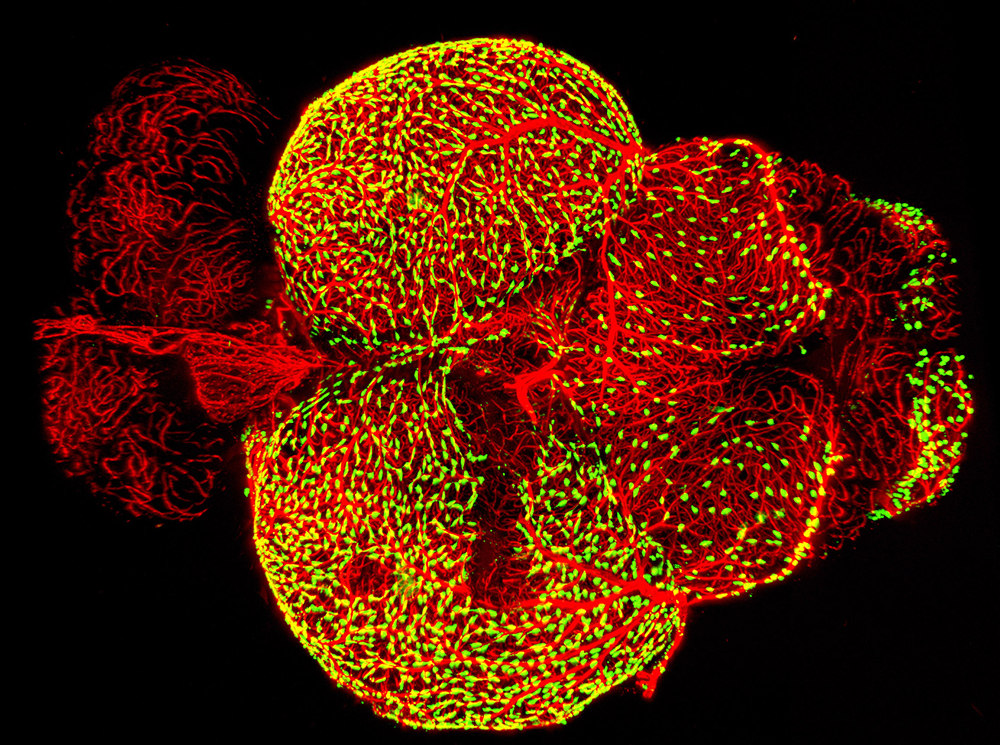
Figure 3. Novel perivascular cells on the zebrafish brain
Confocal micrograph of fluorescent granular perithelial cells (FGPs, green) adhering to the outside of meningeal blood vessels (red) on the brain of a Tg(mrc1a:egfp); Tg(kdrl:cherry) double-transgenic adult zebrafish. We recently showed that FGPs are unique endothelium-derived perivascular cells with unusual scavenging properties that are likely to be critical for brain homeostasis.
Specification and patterning of the lymphatic system
The lymphatic system is a vascular system completely separate from the blood circulatory system and comprises an elaborate blind-ended tree of vessels that extensively innervate most of the body, emptying lymph fluid into the venous blood vascular system via several evolutionarily conserved drainage points. The lymphatic system is essential for immune responses, fluid homeostasis, and fat absorption, and is involved in many pathological processes, including tumor metastasis and lymphedema. However, progress in understanding the origins and early development of the system has been hampered by difficulties in observing lymphatic cells in vivo and performing defined genetic and experimental manipulation of the lymphatic system in currently available model organisms. Our groundbreaking studies demonstrated that zebrafish possess a lymphatic system that shares many of the morphological, molecular, and functional characteristics of lymphatic vessels found in other vertebrates, providing a powerful model for the purpose of imaging and studying lymphatic development. We are currently pursuing further study of the formation of the lymphatic system through several ongoing projects.
- We generated new transgenic lines that permit direct, specific visualization of the developing lymphatic vasculature and are using sophisticated imaging of these transgenic animals to characterize lymphatic development (Figure 4).
- We carried out forward-genetic ENU (N-ethyl-N-nitrosourea) mutagenesis screens using our lymphatic reporter transgenic lines to identify new lymphatic-specific mutants with defects in novel genes that play important roles in lymphatic development.
- We are characterizing and studying novel microRNAs expressed in the lymphatic endothelium and how these small regulatory RNAs influence lymphatic gene expression and lymphatic development.
- We are studying the formation of previously uncharacterized lymphatic vascular networks surrounding the zebrafish brain. Like similar brain lymphatic vessels recently discovered in the mammalian brain, the zebrafish vessels are likely to play critical roles in maintaining homeostasis and protecting the brain, and we are carrying out a detailed analysis of the development, form, and function of these critical vessels.
The results of our studies, combining the genetic and experimental tools available in the zebrafish with the ability to perform high-resolution microscopic imaging of developing vascular structures in living animals, will continue to lead to important new insights into the origins and growth of the lymphatic system and molecular mechanisms that are critical in lymphatic development and lymphatic pathologies.
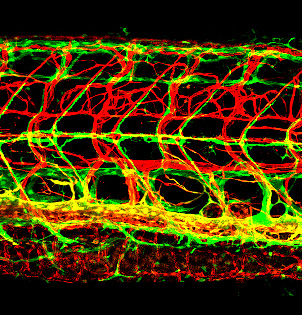
Figure 4. Novel lymphatic vascular reporter
Lateral view confocal image of the trunk of a 12 dpf (days post-fertilization) Tg(kdrl:cherry); Tg(mrc1a:egfp) double-transgenic zebrafish with red fluorescent blood vessels and green fluorescent lymphatics. See Jung HM, et al. Development 2017;144:2070 for additional details.
Epigenetics of development
We are using the genetically and experimentally accessible zebrafish and Mexican tetra (Astyanax mexicanus) models to uncover the molecular basis for organ- and tissue-specific epigenetic regulation during development in the following interrelated projects.
Epigenetic regulation of fat and muscle development in cavefish
In addition to eye and pigment loss and other adaptations, Astyanax cavefish (Figure 5) have extreme and unusual metabolic adaptations that allow them to survive chronic and long-term food deprivation, including excess fat deposition, altered liver function, and resistance to metabolic disease. We hypothesize that, in a similar manner to loss of eyes, changes in epigenetic gene regulation may also underlie cavefish metabolic adaptations. We are using single-cell profiling to investigate differences in adipocytes and other cell types in the muscles (where in cavefish there are large amounts of fat stored) and livers of cavefish and surface fish. We are also performing whole-genome bisulfite sequencing and RNA-Seq from surface and cavefish muscles and livers to identify differentially expressed and methylated genes. We will follow up on these findings to elucidate how differential DNA methylation influences fat metabolism and obesity.
Figure 5. Mexican tetra cave and surface fish
The Mexican tetra Astyanax mexicanus is a freshwater fish native to parts of southern Texas and eastern and central Mexico, which exists in both surface-dwelling (“surface morphs,” top right) and very closely related cave-dwelling (“cave morphs,” bottom left) populations. Cave morphs have a series of uniquely evolved adaptations including loss of eyes and pigment, dramatically altered metabolism, altered vascular function, and altered sleep regulation and behavior. Results from our laboratory suggest that altered DNA methylation and resulting coordinated changes in expression of large sets of genes have helped drive at least some of this rapid evolutionary change.
Uncovering molecular mediators of glycemic memory in diabetic vasculopathy
The global burden of diabetes has risen dramatically, with projections that more than 600 million adults will be affected by 2030. Micro- and macrovascular complications in patients with diabetes are the major causes of cardiovascular mortality, renal failure, blindness, and non-traumatic amputations. Diabetes-related complications can emerge even many years after the blood sugar level levels have been brought under control, a phenomenon known as “glycemic memory.” Although the cause of the phenomenon remains to be elucidated, epigenetic alterations in endothelial cells (ECs) may be responsible for the perdurance of diabetic vascular effects. We are using the zebrafish as an in vivo model to examine whether short-term exposure to hyperglycemia results in persistent transcriptomic and epigenomic changes in endothelial cells, even after return to normoglycemic conditions. We identified several genes with significantly altered endothelial transcription and methylation levels during hyperglycemia that persist during the memory phase. We are currently carrying out further investigation of such “glycemic memory loci” using CRISPR genome editing and other methods. Unveiling the epigenetic and transcriptomic landscape of glycemic memory in ECs may lead to better identification of molecular targets and, potentially, to the design of personalized, epigenetic-based therapies to alleviate the enormous burden of diabetic vasculopathy.
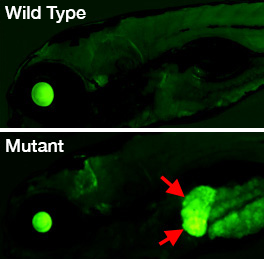
Forward-genetic screen for epigenetic regulatory factors
Genetic screens carried out in Drosophila and the nematode Caenorhabditis elegans have been highly successful in identifying genes regulating cell type–specific epigenetic gene regulation in invertebrates, but the molecular mechanisms involved in organ- and tissue-specific epigenetic regulation in vertebrates are still relatively unknown. We developed a novel zebrafish transgenic reporter line that allows us to monitor dynamic changes in epigenetic regulation in intact animals during development. Using the transgenic line, we are performing the first large-scale F3 genetic screen in a vertebrate to identify recessive mutants in regulators of epigenetic gene silencing or activation (Figure 6).
Figure 6. An epigenetic silencing mutant in the zebrafish
Lateral views of the head and anterior trunk of a wild-type (top) and tissue-specific epigenetic silencing mutant (bottom) zebrafish. The mutant causes loss of epigenetic silencing specifically in the liver (red arrows), as visualized with a novel transgenic reporter line developed in our lab, which permits dynamic, tissue-specific visualization of epigenetic silencing in living animals.
Additional Funding
- Intramural Aids Research Fellowship (to M. Venero Galanternik)
- K99/R00 Award (to M. Venero Galanternik)
- Japan Society for the Promotion of Science (JSPS) Award (to K. Taimatsu)
- NICHD Intramural Research Fellowship (to L. Greenspan)
Publications
- Ma L, Gore AV, Castranova D, Shi J, Ng M, Tomins KA, van der Weele CM, Weinstein BM, Jeffery WR. A hypomorphic cystathionine ß-synthase gene contributes to cavefish eye loss by disrupting optic vasculature. Nat Commun 2020;11:2772.
- Stratman AN, Farrelly OM, Mikelis CM, Miller MF, Wang Z, Pham VN, Davis AE, Burns MC, Pezoa SA, Castranova D, Yano JJ, Kilts TM, Davis GE, Gutkind JS, Weinstein BM. Anti-angiogenic effects of VEGF stimulation on endothelium deficient in phosphoinositide recycling. Nat Commun 2020;11:1204.
- Stratman AN, Weinstein BM. Assessment of vascular patterning in the zebrafish. Methods Mol Biol 2020;2206:205-222.
- Li D, March ME, Gutierrez-Uzquiza A, Kao C, Seiler C, Pinto E, Matsuoka LS, Battig MR, Bhoj EJ, Wenger TL, Tian L, Robinson N, Wang T, Liu Y, Weinstein BM, Swift M, Jung HM, Kaminski CN, Chiavacci R, Perkins JA, Levine MA, Sleiman PMA, Hicks PJ, Strausbaugh JT, Belasco JB, Dori Y, Hakonarson H. ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nat Med 2019;25:1116-1122.
- Jung HM, Hu CT, Fister AM, Davis AE, Castranova D, Pham VN, Price LM, Weinstein BM. MicroRNA-mediated control of developmental lymphangiogenesis. eLife 2019;8:e46007.
Collaborators
- Andreas Baxevanis, PhD, Computational and Statistical Genomics Branch, NHGRI, Bethesda, MD
- Harold Burgess, PhD, Section on Behavioral Neurogenetics, NICHD, Bethesda, MD
- George Davis, PhD, University of Missouri-Columbia, Columbia, MO
- Elisabetta Dejana, PhD, The FIRC Institute of Molecular Oncology Foundation, Milan, Italy
- Silvio Gutkind, PhD, Oral and Pharyngeal Cancer Branch, NIDCR, Bethesda, MD
- James Iben, PhD, Molecular Genomics Laboratory, NICHD, Bethesda, MD
- Sumio Isogai, PhD, Iwate Medical University, Morioka, Japan
- William R. Jeffery, PhD, University of Maryland, College Park, MD
- Paul Liu, MD, PhD, Genetics and Molecular Biology Branch, NHGRI, Bethesda, MD
- Richard Maraia, MD, Section on Molecular and Cell Biology, NICHD, Bethesda, MD
- Yoh-suke Mukouyama, PhD, Laboratory of Stem Cell and Neuro-Vascular Biology, NHLBI, Bethesda, MD
- Lisa M. Price, PhD, Division of Developmental Biology, NICHD, Bethesda, MD
- Radu V. Stan, MD, PhD, Geisel School of Medicine at Dartmouth, Lebanon, NH
Contact
For more information, email weinsteb@mail.nih.gov or visit http://uvo.nichd.nih.gov.