Physiological, Biochemical, and Molecular-Genetic Events Governing the Recognition and Resolution of RNA/DNA Hybrids

- Robert J. Crouch, PhD, Head, Section on Formation of RNA
- Susana M. Cerritelli, PhD, Staff Scientist
- Kiran Sakhuja, MS, MSc, Biologist
- Yasmeen Ajaj, BS, Postbaccalaureate Fellow
- Emily Helm, BA, BS, Postbaccalaureate Fellow
- Xiang Zheng, BS, Postbaccalaureate Fellow
Damaged DNA is a leading cause of many human diseases and disorders. We study the formation and resolution of RNA/DNA hybrids, which occur during DNA and RNA synthesis. Such hybrid molecules may lead to increased DNA damage but may also play critical roles in normal cellular processes. We are interested in how RNA/DNA hybrids are resolved and in the role that ribonucleases H (RNases H) play in their elimination. Two classes of RNases H, Class I and Class II, are present in most organisms.
Human patients with mutations in RNASEH1 exhibit a typical mitochondrial myopathy phenotype (muscular). Our studies were the first to show that RNase H1 is essential for the maintenance of mitochondrial DNA. Mice deleted for the Rnaseh1 gene arrest embryonic development at day 8.5 as a result of failure to amplify mitochondrial DNA. Aicardi-Goutières syndrome (AGS), a severe neurological disorder with symptoms appearing at or soon after birth, can be caused by defective human RNase H2. We are examining mouse models of AGS to gain insight into the human disorder. To understand the mechanisms, functions, substrates, and basic molecular genetics of RNases H, we employ molecular-genetic and biochemical tools in yeast and mouse models.
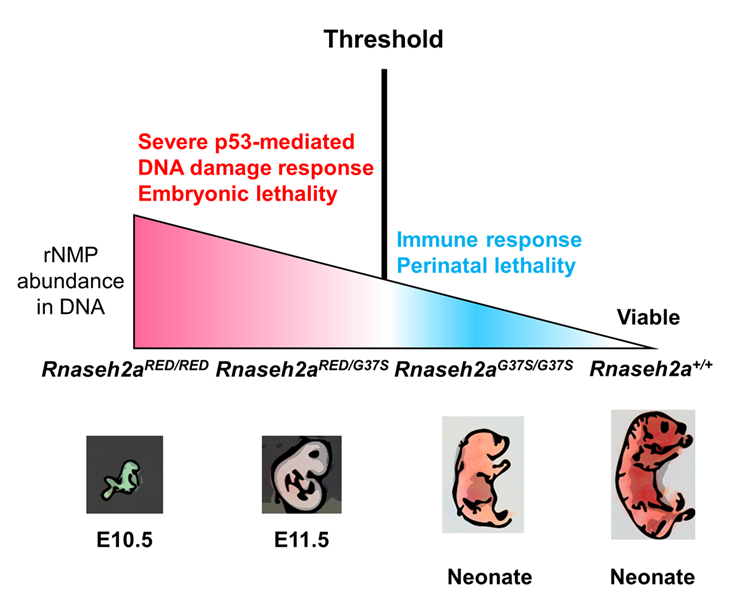
Figure 1. Two RNase H2 mutants with differential ribonucleotide excision repair activity reveal a threshold of rNMPs for embryonic development.
RNase H2 has two distinct functions: initiation of the ribonucleotide excision repair (RER) pathway by cleaving ribonucleotides (rNMPs) incorporated during DNA replication; and processing the RNA portion of an R-loop formed during transcription. An RNase H2 mutant that lacked RER activity but supported R-loop removal revealed that rNMPs in DNA initiate the p53–dependent DNA damage response and cause early embryonic arrest in the mouse. However, an RNase H2 AGS–related mutant with residual RER activity develops to birth. Estimations of the number of rNMPs in DNA in these two mutants define a ribonucleotide threshold above which p53 induces apoptosis. Below the threshold, rNMPs in DNA trigger an innate immune response. We are currently examining HEK293 cells with mutations in the RNASEH2A gene, which yield RNase H2 proteins with a preference for incision at single rNMPs embedded in DNA and poor activity toward R-loops.
Differences between Class I and Class II RNases H
Many of our investigations over the years focused on RNase H1. RNase H1 recognizes the 2′-OH of four consecutive ribonucleotides, while the DNA strand is distorted to fit into a pocket of the enzyme. Thus, the enzyme requires more than one ribonucleotide for cleavage of RNA in RNA/DNA hybrids. In both eukaryotes and prokaryotes, RNases H1 consist of a single polypeptide. In contrast, RNase H2 is a complex of three distinct polypeptides in eukaryotes but a single polypeptide in prokaryotes. The catalytic subunit of the hetero-trimeric RNase H2 of eukaryotes is similar in its primary amino-acid sequence to the prokaryotic enzyme. RNase H2 can recognize and cleave both RNA/DNA hybrids and a single ribonucleotide or the transition from the ribonucleotide in the case of RNA–primed DNA synthesis (e.g., rrrrrDDDD in DNA—italics indicate transition from ribonucleotide to deoxyribonucleotide) [References 1 & 2].
Several types of RNA/DNA hybrid structure are formed, and they are processed differently. Simple RNA/DNA hybrids consist of one strand of RNA paired with one strand of DNA. The HIV–AIDS reverse transcriptase (RT) forms such hybrids when copying its genomic RNA into DNA. The RT also has an RNase H domain that is structurally and functionally similar to the class I cellular RNase H and is necessary for several steps of viral DNA synthesis. R-loop hybrids (three-stranded nucleic acid structures) have two separated DNA strands, with one hybridized to RNA while the other is in a single-stranded form. Such structures sometimes form during transcription and can lead to chromosomal breakage. However, they are also part of the normal process of switching (recombination) from one form of immunoglobulin to another, resulting in distinct isoforms of antibodies. Another form of hybrid are single or multiple ribonucleotides incorporated into DNA during replication [Reference 1]. The first two types of hybrid are substrates for class I and II RNases H. The third is uniquely recognized by type 2 RNases H.
Dual activities of RNase H2; Aicardi-Goutières syndrome
Eukaryotic RNases H2 recognize and resolve RNA hybridized or covalently attached to DNA (two chemically distinct structures) using the same catalytic mechanism for hydrolysis. RNase H2 mutations that reduce catalytic activity, or fail to properly interact with in vivo substrates, cause Aicardi-Goutières syndrome (AGS). Mutations in seven genes are known to cause AGS, with more than 50% of AGS patients having mutations in any of the three subunits of RNase H2. We previously expressed (in Escherichia coli) and purified human RNases H2 with mutations corresponding to several of those seen in AGS patients; one such mutation, RNASEH2A–G37S (G37S), has significant loss of RNase H2 activity. Using the 3D structure of the human enzyme that we had determined, we could locate all known mutations in RNase H2 that cause AGS. The wide distribution of the mutations suggests that modest changes in stability and interaction with other unknown proteins, as well as loss of catalysis, can all cause AGS. A mutation near the catalytic center of G37S found in some AGS patients results in low RNase H2 activity for both embedded ribonucleotides in DNA and RNA/DNA hybrids [Reference 1]. We are developing mouse models of AGS to clarify which defects are associated with each RNase H2 activity.
Mice bearing the G37S mutation in homozygous form are perinatal lethal, i.e., either dead at birth or die within a few hours of birth [Reference 1]. Mutations in another gene, TREX1, also cause AGS, and it has been shown that homozygous knockout (KO) mice are viable but die after a few weeks owing to a cardiomyopathy that can be prevented by blocking either an innate or adaptive immune response. In contrast, the G37S–mutant perinatal lethality and the fact that RNase H2 KO mice die during early embryogenesis suggest a more severe defect than that seen in TREX1–KO mice. Damaged DNA that finds its way into the cytoplasm can be sensed by the cGAS protein producing the small molecule cGAMP, which interacts with the Sting protein, an important protein for the DNA–sensing in the innate immune pathway. Mice that are homozygous for G37S and deleted for the cGAS or Sting genes are mostly perinatal lethal but no longer exhibit increases in ISGs (interferon-stimulated genes). Interestingly, a small fraction of the double G37S–Sting KO are viable, indicating only limited involvement of ISGs in perinatal lethality. Further studies are under way, which we expect will lead us to the cause of lethality.
To distinguish among the defects that persistent RNA/DNA hybrids and single ribonucleotides joined to DNA cause in vivo, Hyongi Chon, a former postdoctoral fellow, rationally designed a modified RNase H2 to make an enzyme unable to cleave single ribonucleotides embedded in DNA but that retained RNA/DNA hydrolytic activity. The mutant enzyme, which we call RED (ribonucleotide excision deficient), resolves RNA/DNA hybrids, which are substrates of both RNase H1 and RNase H2. Unlike the mouse and human RNases H2, RNase H2 activity is not required in the yeast Saccharomyces cerevisiae. Employing the ease of genetic mutation studies in yeast, we demonstrated that yeast producing the RNase H2RED enzyme acted in vivo by leaving embedded ribonucleotides (rNMPs) in DNA but was potent in removing RNA in RNA/DNA hybrids.
Embryonic lethality of mice Rnaseh2b–KO strains has been attributed to accumulation of rNMPs in DNA, but lethality could be the result of loss of RNA/DNA hydrolysis or a combination of both rNMP and RNA/DNA hydrolysis defects [References 1 & 2]. To distinguish among the possible causes of embryonic lethality, we generated a mouse that produces the RNase H2RED enzyme. Mouse embryonic fibroblasts (MEFs) derived from Rnaseh2RED mice have the same high level of rNMPs as seen in Rnaseh2b–KO MEFs [Reference 2]. Interestingly, the Rnaseh2RED mice die around the same time as the Rnaseh2b–KO mice. Therefore, lethality of the Knockout and RED RNase H2 mouse strains may be caused by increased rNMPs in genomic DNA. Rnaseh2aG37S/RED embryos also arrest at approximately the same stage as Rnaseh2aRED/RED embryos because of better association of RNase H2RED than RNase H2G37S with DNA substrate containing embedded rNMPs. The result is important because some RNase H2–AGS patients have similar compound heterozygous mutations in which there may be a dominant mutated enzyme.
Our studies on RNase H2-RED have permitted us and others to assign specific substrates to each of the two activities and determine which functions are related to various phenotypes seen when RNase H2 is absent [References 1—5]. One of our goals is to produce an RNase H2 with robust incision at single rNMPs in DNA but with poor RNA/DNA hybrid cleavage (hybrid-defective [HD]) to complement the RNase H2-RED enzyme. We have identified amino-acid changes that appear to produce such RNase H2—HD and are in the early stages of studies in both cell cultures and mice.
Detection of a threshold of ribonucleotide tolerance in DNA for embryonic development
Embryonic development in the absence of RNase H2 exhibits defects as early as E6.5 to 7.5, the period of gastrulation in which cell numbers double every 4–5 h. We found evidence that this is indeed the cause of embryonic lethality [Reference 2]. We used mice with a separation of function in the RNase H2 enzyme (RNase H2RED) that retained RNA/DNA hydrolysis but was unable to remove rNMPs in DNA. Embryonic development was arrested at E10, the same day as seen for embryos with complete loss of both of RNase H2’s functions. Compared with complete loss of both functions, RNase H2RED/RED mouse embryo fibroblast cells have only about 65% rNMPs in DNA. A mouse (RNase H2G37S) with partial loss of both RNase H2 activities develops to birth and retains about 30% of the number of rNMPs in DNA compared with cells with complete loss of RNase H2. A compound heterozygous mouse, in which both RNase H2RED and RNaseh2G37S are present, retains 40% of rNMPs in cells lacking RNase H2. Embryos with complete loss of RNase H2 or that express RNase H2RED or RNase H2RED/G37S all exhibit a p53–dependent DNA–damage response. In contrast, mice with RNase H2G37S develop to birth with little or no p53–dependent DNA damage. Mice expressing RNase HG37S weigh about 1,000 mg at birth, whereas the early-arrested embryos with no RNase H2 or defective RNases H2-RED or -RED/G37S weight only one to a few mg, an enormous difference. We conclude that a threshold of tolerance of rNMPs in DNA for embryonic development past E10 is exceeded in all mouse strains tested except in RNase HG37S.
Human patients with the RNASEH2A G37S mutation have AGS. Although the patients with RNASEH2A G37S mutations are homozygous, similar to our RNase H2RED/G37S mice, some AGS patients are compound heterozygous, with each allele having a different mutation in the same RNase H2 gene. In vitro studies of mutant forms of RNase H2 reflect the properties of the RNase H2 mutant but may be unreliable for assessing the contribution of each of the two forms of RNase H2 when both are present in vivo. The strong effect on the stage of lethality of RNase H2RED/G37S embryos indicates a competition between the rNMP–active RNase H2G37S and the inactive RNase H2RED for some step in the removal of rNMPs. The protein-proliferating cell nuclear antigen (PCNA) is a critical component for removing rNMPs in DNA. We suggest that the competition between RNase H2-RED and RNase H2-G37S occurs when RNase H2 interacts with PCNA to repair rNMPs in DNA, rather than binding to rNMPs in DNA.
High density of unrepaired genomic ribonucleotides leads to Topoisomerase 1–mediated severe growth defects in absence of ribonucleotide reductase in Saccharomyces cerevisiae.
DNA polymerases incorporate rNMPs during replication and repair, and the rate of incorporation is affected by the ratio of deoxyribonucleotides (dNTPs)/ribonucleotide (rNTPs) in cells. We reduced the cellular pools of dNTPs in yeast by depleting Rnr1, the major catalytic subunit of ribonucleotide reductase (RNR), which converts ribonucleotides to deoxyribonucleotides, and observed an accumulation of genomic rNMPs in absence of RNase H2. Deletion of the genes for RNase H1 and the catalytic subunit of RNase H2 under Rnr1 depletion induced cell lethality, presumably because cells cannot survive the combination of high levels of rNMPs in DNA and persistent RNA/DNA hybrids, leading to replicative stress, DNA damage, and cell death. We further increased the load of rNMPs in genomic DNA by using DNA polymerases with higher propensity to incorporate rNMPs during DNA synthesis and observed that cells lacking both Rnr1 and RNase H2 suffer from severe growth defects, defects that are reversed in absence of Topoisomerase 1 (Top1), an enzyme that transiently breaks and reseals phosphodiester bonds in DNA, because processing of rNMPs by Top1, when RER is defective, induces mutations and genome instability. We concluded that, in cells lacking RNase H2 and containing a limiting supply of dNTPs, there is a threshold of tolerance for the accumulation of genomic ribonucleotides that is tightly associated with Top1–mediated DNA damage.
Our studies on RNase H2-RED permitted us and others to assign specific substrates to each of the two activities and to determine which functions are related to various phenotypes seen when RNase H2 is absent [References 1–5]. One of our goals is to produce an RNase H2 with robust incision at single rNMPs in DNA but with poor RNA/DNA hybrid cleavage (Hybrid defective, HD) to complement the RNase H2-RED enzyme. We identified amino-acid changes that appear to produce such RNase H2-HD, and we are in the early stages of studies in both cell cultures and mice.
Abasic substrates
Incorporated rNMPs embedded in DNA could be converted to abasic sites in which the flanking dNMPs would be connected by a ribose phosphate rather than an rNMP. In collaboration with the Storici and Tell groups, we examined the abilities of eukaryotic RNases H2 to cleave substrates containing a single ribose abasic (only sugar phosphate) site in duplex DNA. Prokaryotic RNases HII, but not eukaryotic RNases H2, can recognize and cleave at rNMPs in duplex DNA. Little was known about abasic sites in RNA until, in collaboration with Cheung’s group, we discovered that abasic sites are present in RNAs of yeast and human cells, and likely in all organisms. The abasic sites are located in or near R-loops. The Cheung group had previously shown that MPG and APE1 interact with R-loops. MPG is a glycosylase that removes the base of DNA, and as reported in our papers with Cheung, or RNA, which in turn can be cleaved by the apurinic/apyrimidinic endonuclease 1 (APE1). Abasic sites in DNA are repaired by Ape1 excision using the complementary DNA strand as template for repair. RNAs have no template to correct for the absence of a base. The association of abasic sites with R-loops is intriguing and suggests that RNAs complexed with DNA are protected from RNase H activities, similar to stable R-loops present at transcription start sites of highly transcribed genes. In theory, the DNA of R-loops could serve as templates for repair of the abasic site, in which case the abasic site would survive longer than abasic sites in single-stranded RNA, giving the appearance of association of abasic sites with R-loops. However, binding of MPG and APE1 suggests that R-loops might be good substrates for MPG to generate the abasic sites.
Publications
- Cerritelli SM, Crouch RJ. RNase H2-RED carpets the path to eukaryotic RNase H2 functions. DNA Repair 2019;84:102736.
- Uehara R, Cerritelli SM, Hasin N, Sakhuja K, London M, Iranzo J, Chon H, Grinberg A, Crouch RJ. Two RNase H2 mutants with differential rNMP processing activity reveal a threshold of ribonucleotide tolerance in DNA for embryonic development. Cell Rep 2018;25:1135-1145.
- Cerritelli SM, Iranzo J, Sharma S, Chabes A, Crouch RJ, Tollervey D, El Hage A. High density of unrepaired genomic ribonucleotides leads to Topoisomerase 1-mediated severe growth defects in absence of ribonucleotide reductase. Nucleic Acids Res 2020;48:4274-4297.
- Liu Y, Rodriguez Y, Ross RL, Zhao R, Watts JA, Grunseich C, Bruzel A, Li D, Burdick JT, Prasad R, Crouch RJ, Limbach PA, Wilson SH, Cheung, VG. RNA abasic sites in yeast and human cells. Proc Natl Acad Sci USA 2020;117:20689-20695.
Collaborators
- Frederic Chedin, PhD, University of California Davis, Davis, CA
- Vivian G. Cheung, MD, University of Michigan Life Sciences Institute, Ann Arbor, MI
- Yosuke Mukoyama, PhD, Laboratory of Stem Cell & Neuro-Vascular Biology, NHLBI, Bethesda, MD
- Keiko Ozato, PhD, Section on Molecular Genetics of Immunity, NICHD, Bethesda, MD
- Francesca Storici, PhD, School of Biological Sciences, Georgia Institute of Technology, Atlanta, GA
- Gianluca Tell, PhD, Università degli Studi di Udine, Udine, Italy
- Kiyoshi Yasukawa, PhD, Kyoto University, Kyoto, Japan
Contact
For more information, email crouch@helix.nih.gov or visit http://sfr.nichd.nih.gov.