Nervous System Development and Plasticity

- R. Douglas Fields, PhD, Head, Section on Nervous System Development and Plasticity
- Philip Lee, PhD, Staff Scientist
- William Huffman, MA, Technician
- Erin Santos, Postbaccalaureate Fellow
- Catherine Carr, PhD, Special Volunteer
Healthy brain and cognitive development in children is central to the mission of the NICHD. Our research is concerned with understanding the molecular and cellular mechanisms by which functional activity in the brain regulates development of the nervous system during late stages of fetal development and early postnatal life. In addition to synaptic plasticity, we are interested in novel mechanisms of activity-dependent nervous system plasticity that are particularly relevant to the period of childhood, including the involvement of glia (non-neuronal brain cells). Our work has three main areas of emphasis: myelination and neuron-glia interactions; cellular mechanisms of learning; and gene regulation by neuronal firing.
Traditionally, the field of activity-dependent nervous system development has focused on synapses, and we continue to explore synaptic plasticity. However, our research is also advancing our understanding of how glia sense neural impulse activity and how activity-dependent regulation of glia contributes to development, plasticity, and the cellular mechanisms of learning. A major emphasis of our current research is to understand how myelin (white matter in the brain) is regulated by functional activity. By changing conduction velocity, activity-dependent myelination may be a non-synaptic form of plasticity, regulating nervous system function by optimizing the speed and synchrony of information transmission through neural networks. Our studies have identified several cellular and molecular mechanisms for activity-dependent myelination, and the findings have important implications for normal brain development, learning, cognition, and psychiatric disorders. Our research shows that myelination of axons by glia (oligodendrocytes in the CNS and Schwann cells in the peripheral nervous system [PNS]) is regulated by impulse activity, and we have identified several molecular mechanisms that control proliferation and differentiation of myelinating glia and myelination. Most recently, we determined that myelin thickness can be adjusted through a treadmilling process that adds and removes layers of myelin from the sheath to adjust conduction velocity and improve functional performance by optimizing spike-time arrival at synapses. The findings provide evidence for a new form of nervous system plasticity and learning that would be particularly important in child development, but which also operates in adulthood, thereby improving function based on experience.
Learning is perhaps the most important function of childhood. Our research is determining the molecular mechanisms that convert short-term memory into long-term memory. If functional experiences produce lasting effects on brain development and plasticity, specific genes must be regulated by specific patterns of impulse firing. We are determining how various patterns of neural impulses regulate specific genes controlling development and plasticity of neurons and glia, and how synaptic strength is modified in the hippocampus.
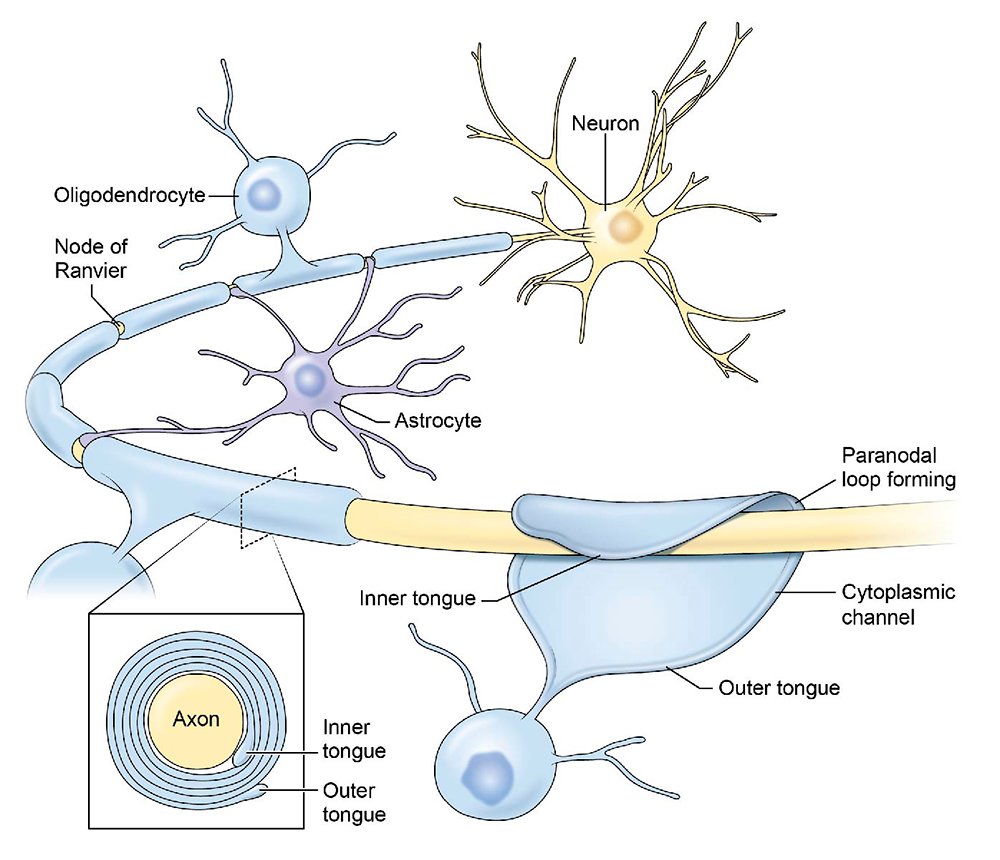
Nervous system plasticity by activity-dependent myelination
Myelin, the multilayered membrane of insulation wrapped around nerve fibers (axons) by glial cells, is essential for proper neural impulse transmission and nervous system function. Myelination is an essential part of brain development, but the processes controlling myelination of appropriate axons are not well understood. Myelination begins in late fetal life and continues throughout childhood and adolescence, but myelination of some brain regions is not complete until an individual's early twenties.
Traditionally myelin has been viewed in terms of conduction failure after damage (for example in multiple sclerosis), but we are exploring how changes in myelin driven by functional activity affect the timing of neural-impulse arrival at synaptic relay points, which is critical for information processing and synaptic activity. In addition, the frequency, phase, and amplitude coupling of oscillations in the brain (brainwaves) requires appropriate impulse conduction velocity, which is influenced by myelination. Many neurological and psychological dysfunctions can develop when optimal neural synchrony of spike-time arrival and neural oscillations are disturbed; for example, in schizophrenia, epilepsy, dyslexia, and autism.
Our research shows that, to activate receptors on myelinating glia as well as on astrocytes and other cells, neurotransmitters are released not only at synapses but also along axons firing action potentials. The recipient cells in turn release growth factors, cytokines, and other molecules that regulate myelination, proliferation, and development of myelinating glia.
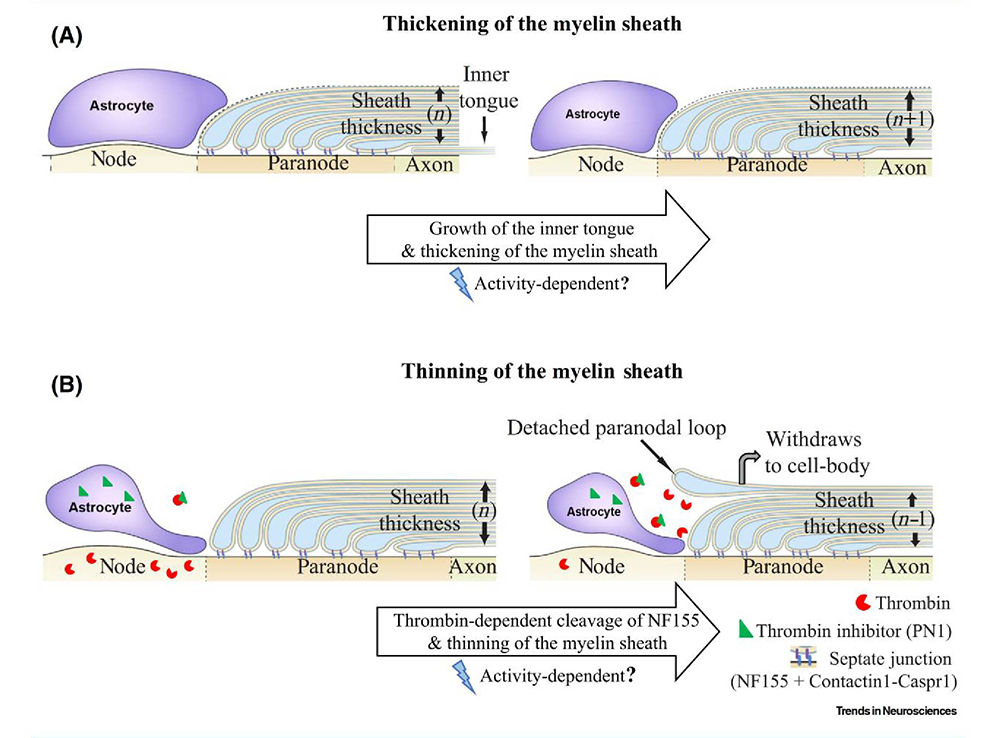
Figure 2. Treadmilling model for plasticity of the myelin sheath
The speed of neural impulse transmission is altered by changes in myelin structure. The thickness of the myelin sheath in central nervous system axons is determined by two opposing processes: one (A) that adds additional wraps of myelin to the axon, and the other (B) that removes the outer layer, thereby increasing and decreasing impulse conduction velocity, respectively. New layers of myelin are added beneath the overlaying layers by expansion of the inner tongue of myelin. Myelin is attached to the axon at the paranodal region flanking the node of Ranvier via septate-junctions, comprised of neurofascin 155 on myelin interacting with Contactin1-Caspr1 complex on the axon. Cleavage of neurofascin 155 by thrombin (red) can break this interaction, resulting in detachment of the outer paranodal loop from the axon, and withdrawal of the outer layer of myelin, which increases nodal gap length and reduces myelin sheath thickness; both effects slow conduction velocity. Perinodal astrocytes at the nodes of Ranvier regulate the process by secreting thrombin inhibitors (green triangle), such as Protease Nexin1. The treadmilling process helps achieve optimal conduction velocity in individual axons [Reference 4].
Induction of myelination by action potentials
In addition to establishing the effects of impulse activity on proliferation and development of myelinating glia, we determined that release of the neurotransmitter glutamate from vesicles along axons triggers the initial events in myelin induction, including stimulating the formation of cholesterol-rich signaling domains between oligodendrocytes and axons and increasing the local synthesis of myelin basic protein, the major protein in the myelin sheath, through Fyn kinase–dependent signaling. We showed that, through this axon-oligodendrocyte signaling mechanism, electrically active axons become preferentially myelinated by a factor of 8 to 1 over electrically inactive axons, thus regulating myelination of axons and neural circuit function according to functional experience, which would be particularly important in the adolescent brain, for example, where environmental experience during sensitive periods can have long-lasting effects on neural circuit development and behavior. The findings are also relevant to such demyelinating disorders as multiple sclerosis and to re-myelination after axon injury.
Modification of myelin structure and conduction velocity by astrocytes
Given that optimal neural-circuit function and synaptic plasticity require the proper impulse transmission speed through all axons to induce spike timing–dependent plasticity and to sustain oscillations at appropriate frequencies, mechanisms that determine and modify conduction time through axons could provide a non-synaptic mechanism of neural circuit plasticity. Conduction velocity in myelinated axons depends on the thickness of the myelin sheath and the morphology of the electrogenic nodes of Ranvier (gaps in the myelin sheath) along axons. Our research showed that myelination of unmyelinated axons and the thickness of the myelin sheath can be increased in response to neural activity and environmental experience. Prior to our research, myelin structure was believed to be static, and there was no known mechanism that could reduce the thickness of the mature myelin sheath (except in the context of pathology). However, a mechanism would be necessary to reduce conduction velocity to achieve optimal spike-time arrival from inputs that arrive at relay points in neural networks too soon.
Our research shows that myelin thickness and nodal gap length are reversibly altered by astrocytes, glial cells that contact nodes of Ranvier, and that this alters the speed of impulse transmission and neural network function. Myelin is attached to the axon by intercellular junctions adjacent to the nodes of Ranvier. We found that one of these cell-adhesion molecules (neurofascin 155) has a binding site for the proteolytic enzyme thrombin, which is secreted by neurons and enters the brain from the vascular system. We found that thrombin-dependent cleavage of neurofascin 155 severs the tether between the axon and myelin, allowing the latter to detach and rendering the myelin sheath thinner. The process is inhibited by vesicular release of thrombin protease inhibitors from perinodal astrocytes. Previously, it was unknown how the myelin sheath could be thinned, and the functions of perinodal astrocytes were not well understood. Our findings uncover a new form of nervous system plasticity in which myelin structure and conduction velocity are adjusted by astrocytes. The thrombin-dependent cleavage of neurofascin 155 may also have relevance to myelin disruption and repair.
Gulf War Illness
After decades of research, there is still no understanding of how a large group of Gulf War veterans became chronically ill with Gulf War Illness. It is believed that exposure to low levels of sarin nerve gas and combinations of organophosphate insecticides, which impair synaptic function, may be responsible. Our discovery that glutamatergic transmission between axons and oligodendrocytes triggers myelination led us to propose that impairments in myelination caused by disrupted neurotransmission from axons to oligodendrocytes may be an underlying cause of Gulf War Illness. Our research shows that proliferation and development of oligodendrocytes is affected in an animal model of Gulf War illness and in cultures of oligodendrocytes exposed to agents such as sarin nerve gas (acetylcholinesterase inhibitors). Perturbations by these agents of axon-glial interactions, which take place through acetylcholine signaling, could have long-lasting consequences in neural network functions underlying many of the symptoms associated with Gulf War Illness, including among others difficulties with working memory, mental focus, and chronic pain. Organophosphate pesticides operate in a similar manner, and exposures to pesticide contamination, especially in childhood, would impair normal development of oligodendrocytes and myelin formation, contributing to cognitive and psychological dysfunctions.
Regulation of gene expression by action potential firing patterns
All information in the nervous system is encoded in the temporal pattern of neural impulse firing. Given that long-lasting changes in the nervous system require regulated gene expression, appropriate patterns of neural impulse firing driving neural plasticity must control transcription of specific genes, a fundamental question central to the processes of experience-dependent plasticity during development and learning. However, little is known about how neural firing patterns regulate gene expression. Our experiments are revealing the intracellular signaling and gene-regulatory networks that respond selectively to appropriate temporal patterns of action-potential firing to generate adaptive responses.
To determine how gene expression in neurons and glia is regulated by impulse firing, we stimulate nerve cells to fire impulses in differing patterns by optogenetics and by delivering electrical stimulation through platinum electrodes in specially designed cell-culture dishes. Live-cell calcium imaging shows that temporal aspects of intracellular calcium signaling are particularly important in regulating gene expression according to neural-impulse firing patterns in normal and pathological conditions. After stimulation, we measured mRNA and protein expression by gene microarrays, quantitative RT-PCR (reverse transcriptase–polymerase chain reaction), RNA-seq (RNA sequencing), Western blot, and immunocytochemistry. The results confirm our hypothesis that precise patterns of impulse activity can increase or reduce expression of specific genes in neurons and glia. Moreover, our research shows that regulation of gene expression in neurons by specific temporal patterns of impulse activity is not a property of special genes; in general, the neuronal transcriptome is highly regulated by the pattern of membrane depolarization, with hundreds of genes differentially regulated by the temporal code of neuronal firing.
We are also pioneering new methods of transcriptional analysis in neurons. The standard approach to analyzing gene expression is by measuring the abundance of tens of thousands specific gene transcripts in cells by microarray or RNA-seq, as described above, but this approach fails to capture the unique feature of transcriptional regulation in neurons. In contrast to other cells responding to external signals that may drive cells to a steady-state equilibrium, transcriptional networks in neurons are continually modulated dynamically by temporally varying action-potential firing frequencies and burst patterns, together with synchrony and phase relationships among populations of interconnected neurons. Such activity may not alter the abundance of a gene transcript significantly; nevertheless, the coordinated activity within transcriptional networks is being modulated dynamically to modify function.
To address this question, we applied a covariance approach using a Pearson correlation analysis, to determine how pairs of genes in mouse dorsal root ganglion (DRG) neurons are coordinately expressed in response to stimulation producing the same number of action potentials in different temporal patterns. Our analysis of 4,728 distinct gene pairs related to calcium signaling, 435,711 pairs of transcription factors, 820 pairs of voltage-gated ion channels, and 86,862 calcium-signaling genes paired with transcription factors, indicates that genes become coordinately activated by distinct action potential firing patterns. Thus, in addition to regulating the expression level of numerous genes, the temporal pattern of action potential firing profoundly modulates how genes are networked in functional pathways.
Our findings provide a deeper understanding of how nervous system development and plasticity are regulated by information coded in the temporal pattern of impulse firing in the brain. The findings are also relevant to chronic pain, as well as to the regulation of nervous system development and myelination by functional activity.
Differences in chromatin structure between neurons and glia
In collaboration with our NICHD colleague, David Clark, our research is revealing fundamental differences in chromatin structure between neurons and glia. Chromatin can be visualized by electron microscopy as regularly spaced ‘beads-on-a-string,’ in which the beads represent nucleosome cores and the string is the intervening linker DNA. Using MNase (micrococcal nuclease digestion) digestion and RNA-seq, we compared the chromatin structure of purified mouse DRG neurons, cortical oligodendrocyte precursor cells (OPCs), and cortical astrocytes. We found that DRG neurons have shorter average nucleosome spacing (approximately 165 base pairs) than either glial cells (OPCs, with approximately 182 base pair spacing) or astrocytes (with approximately 183 base pairs). The significance of such basic differences in chromatin structure between DRG neurons and these glial cells is unknown and is currently being investigated. Interestingly, the atypical nucleosome spacing of neuronal chromatin does not extend to promoter-proximal regions.
Synaptic plasticity
It is widely appreciated that there are two types of memory, short-term and long-term, and that sleep plays a critical role in memory consolidation. Gene expression is necessary to convert short-term into long-term memory, and our research concerns how signals reach the nucleus to initiate the process and which genes control strengthening and weakening of synapses in association with learning. Long-term potentiation (LTP) and long-term depression (LTD) are two widely studied forms of synaptic plasticity that can be recorded electrophysiologically in the hippocampus and are believed to represent a cellular basis for memory. We use electrophysiology, cDNA microarrays, RNA-seq, calcium imaging, and two-photon in vivo imaging to investigate the signaling pathways, genes, and proteins involved in LTP and LTD in primary cell culture and hippocampal brain slice. The work is contributing to a better understanding of how regulatory networks are controlled by appropriate patterns of impulses, leading to different forms of synaptic plasticity, and is identifying new molecular mechanisms regulating synaptic strength.
Publications
- Dutta DJ, Woo DH, Lee PR, Pajevic S, Bukalo O, Huffman WC, Wake H, Basser PJ, Sheikhbahaei S, Lazarevic V, Smith JC, Fields RD. Regulation of myelin structure and conduction velocity by perinodal astrocytes. Proc Natl Acad Sci USA 2018;115(46):11832-11837.
- Kato D, Wake H, Lee PR, Tachibana Y, Ono R, Sugio S, Tsuji Y, Tanaka YH, Tanaka YR, Masamizu Y, Hira R, Moorhouse AJ, Tamamaki N, Ikenaka K, Matsukawa N, Fields RD, Nabekura J, Matsuzaki M. Motor learning requires myelination to reduce asynchrony and spontaneity in neural activity. Glia 2020;68(1):193-201.
- Iacobas DA, Iacobas S, Lee PR, Cohen JE, Fields RD. Coordinated activity of transcriptional networks responding to the pattern of action potential firing in neurons. Genes (Basel) 2019;10(10):pii E754.
- Fields RD, Dutta DJ. Treadmilling model for plasticity of the myelin sheath. Trends Neurosci 2019;42(7):443-447.
- Lee PR, Fields RD. Activity-dependent gene expression in neurons. Neuroscientist 2020;1073858420943515 (online ahead of print).
Collaborators
- Peter J. Basser, PhD, Section on Quantitative Imaging and Tissue Sciences, NICHD, Bethesda, MD
- David Clark, PhD, Section on Chromatin and Gene Expression, NICHD, Bethesda, MD
- Dumitru Iacobas, PhD, New York Medical College, Valhalla, NY
- Vanja Lazarevic, PhD, Experimental Immunology Branch, Center for Cancer Research, NCI, Bethesda, MD
- James O'Callaghan, PhD, CDC Distinguished Consultant, NIOSH, and West Virginia University, Morgantown, WV
- Sinisa Pajevic, PhD, Division of Computational Bioscience, CIT, NIH, Bethesda, MD
- Shahriar Sheikhbahaei, PhD, Cellular and Systems Neurobiology Section, NINDS, Bethesda, MD
- Jeffrey C. Smith, PhD, Cellular and Systems Neurobiology Section, NINDS, Bethesda, MD
- Kimberly Sullivan, PhD, Boston University School of Public Health, Boston, MA
- Hiroaki Wake, PhD, National Institute for Basic Biology, Okazaki, Japan
Contact
For more information, email fieldsd@mail.nih.gov or visit http://nsdps.nichd.nih.gov.