Signaling and Secretion in Neuroendocrine Cells

- Stanko S. Stojilkovic, PhD, Head, Section on Cellular Signaling
- Yuta Moshimaru, PhD, Visiting Fellow
- Rafael M. Previde, PhD, Visiting Fellow
- Kosara Smiljanic, PhD, Visiting Fellow
- Srdjan J. Sokanovic, PhD, Visiting Fellow
- Kai Wang, PhD, Visiting Fellow
The main goal of the research in our Section is to examine cell-signaling cascades, gene expression, and hormone secretion in neuroendocrine cells from the hypothalamus and pituitary gland during development. We place special emphasis on characterization of individual cells, using fluorescence imaging, patch-clamp recordings, simultaneous membrane potential/calcium and current/calcium recordings, electrophysiological and imaging recordings of single-cell exocytic events, single-cell RNA sequencing (scRNASeq), and single-cell quantitative reverse transcription polymerase chain reaction (RT-PCR). Our recent and ongoing work has focused on signaling, transcription, and secretion in the pituitary gland specific for age, sex, and tissue structure; pituitary cell heterogeneity, reflecting their postnatal gene expression; the role of 1-phosphatidylinositol 4-kinases and protein receptor tyrosine N2 phosphatase type in postnatal proliferation and maintenance of pituitary lineages; as well as cell-specific electrical activity and exocytic pathways. Current and proposed studies depend in part on the use of equipment in NICHD's Microscopy and Imaging Core Facility and the Molecular Genomics Core Facility.
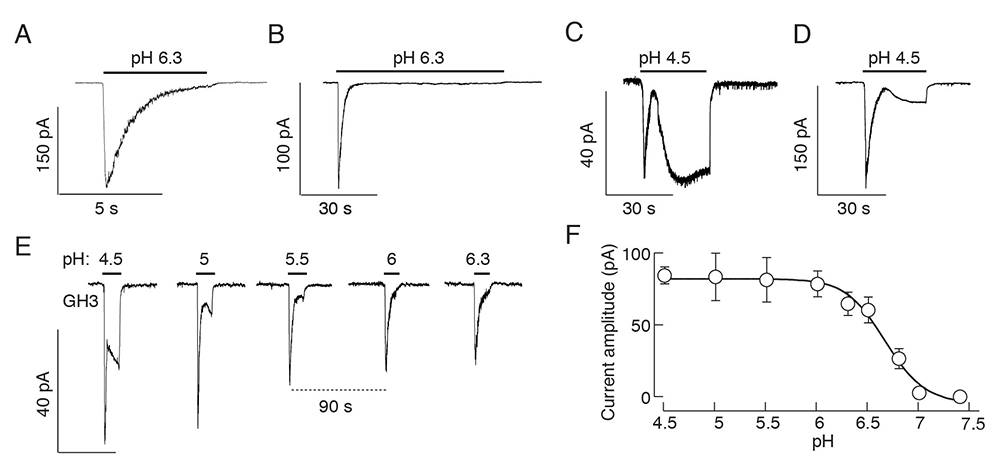
Figure 1. Effects of a drop in extracellular pH on current responses in immortalized GH3 cells
A-D. Patterns of proton-activated currents in voltage-clamped single cells: a biphasic response composed of a transient spike phase and a sustained small amplitude plateau phase during long-term stimulation with pH 6.3 (A and B), and two-current responses to pH 4.5 (C and D) in different cells.
E and F. Proton concentration dependence of the peak amplitudes of fast and slow current responses; representative traces from a single cell during repeated proton application (E) and mean ± SEM values derived from 5–10 single-cell recordings per dose (F). Traces shown are representative of 6 to 10 cells per experiment.
Enhancement of analysis of scRNASeq data: pituitary cell example
The scRNASeq investigation of the pituitary required the development or adaptation of methods for data analysis. The main problem in the analysis was related to the fact that several highly expressed transcripts, including those of Gh1, Prl, Pomc, Lhb, and Tshb, also showed the presence at lower levels in cell types known not to expressed them, for example Gh1 in erythrocytes and Hbb in somatotrophs. To clarify the nature of this background, we added HEK293 cells expressing a GFP–tagged protein to pituitary glands, dispersed the cells, and examined Gh1 expression by single-cell qRT–PCR (quantitative RT-PCR). The analysis showed that all HEK293 cells from such a preparation, but not the control cells, were Gh1–positive, indicating that RNA released from broken cells during dispersion binds extracellularly to neighboring cells, resulting in contamination. To overcome the problem, we developed a threshold calculation method for each gene based on the Otsu’s method to define whether the gene is expressed above background levels. Combined with standard quality-control techniques, such as the removal of potentially damaged cells based on a high proportion of mitochondrial transcripts, the threshold allowed us to develop a logic-based cell-type classification method using marker genes. We also developed a pituitary-specific clustering program for scRNASeq, based on integration of the major cell type–specific genes, along with a method for identification of marker and dominant genes, and separating proliferative from differentiated cells. Such methodological achievements will be highly useful in our current and future work with scRNASeq analysis of rat and human pituitary cells, where the separation of undifferentiated, proliferative, and differentiated cell types, including the presence of bihormonal cells, is essential [Reference 1].
Dependence of gonadotroph marker-gene expression on GnRH application patterns and animal immune status
Mammalian reproduction depends on the proper synthesis and release of two gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), by specialized anterior pituitary endocrine cells called gonadotrophs. The hormones are dimeric glycoproteins composed of a common glycoprotein hormone, α polypeptide (Cga), and unique β subunits (Lhb and Fshb), which give biological specificity. Gonadotropin secretion is tightly regulated by hypothalamic and intrapituitary factors. Among them, the most important is gonadotropin-releasing hormone (GnRH), which is released in a pulsatile manner by a small set of neurons within the preoptic area and the mediobasal hypothalamus. Upon reaching the anterior pituitary, GnRH binds to its receptor expressed in gonadotrophs and signals through a Gq/11–dependent cascade (Gq/11 is a heterotrimeric guananine nucleotide [G]–binding protein) of intracellular pathways that culminate in periodic gonadotropin secretion. The pulsatile pattern of GnRH release is crucial for proper gonadotropin synthesis and release. Several physiological responses and pathological conditions that cause reproductive failure in humans have been associated with impaired regulation of pulsatile GnRH release, including functional hypothalamic amenorrhea, hyperprolactinemia, polycystic ovary syndrome, and hypogonadotropic hypogonadism. Continuous treatment with a GnRH receptor agonist is also clinically relevant, for example, for the prevention of ovarian hyperstimulation syndrome during assisted reproduction, for ovarian protection during chemotherapy, and for the treatment of precocious puberty and polycystic ovary syndrome.
We recently assessed the time course of expression of the gonadotroph marker genes Fshb, Lhb, Gnrhr, and Cga in static cultures of rat anterior pituitary during continuous treatment of GnRH or its analogs. We also studied the expression of the transcription factor genes Egr1, Nr5a1, and Pitx1, which are critical for the control of Lhb expression. In addition, we characterized the acute and long-term effects of cell dispersion and primary culture in the absence of GnRH for these genes and commonly used reference genes, before examining the effect of continuous GnRH treatment. Further, we studied the relationship between Lhb expression, LH cell content, and LH release in vitro during continuous GnRH treatment. We also evaluated the effects of in vivo injection of a GnRH–receptor agonist and antagonist on gonadotropin subunit gene expression and serum and pituitary LH content. We used the expression of two SIBLING genes (a subfamily of the secreted calcium-binding phosphoproteins), Dmp1 and Spp1, which we recently characterized, as internal controls for in vitro and in vivo experiments.
Culturing of pituitary cells in GnRH–free conditions reduced Fshb, Cga, and Gnrhr expression, while continuous treatment with GnRH agonists upregulated Cga expression progressively and Gnrhr and Fshb expression transiently, which was accompanied by a prolonged blockade of Fshb but not Gnrhr expression. In contrast, Lhb expression was relatively insensitive to the loss of endogenous GnRH and continuous treatment with GnRH, probably reflecting the expression status of Egr1 and Nr5a1. We observed similar response patterns in vivo after administration of GnRH agonists. However, continuous treatment with GnRH stimulated LH secretion in vitro and in vivo, leading to a reduction in LH cell content despite high basal Lhb expression. The data suggest that blockade of Fshb expression and depletion of the LH secretory pool are two major factors accounting for weakening of the gonadotroph secretory function during continuous GnRH treatment [Reference 2].
Multiple sclerosis develops in a sex-specific manner during reproductive years. Various neuroendocrine changes in this inflammatory, demyelinating, and debilitating disease have been described. Recently, we aimed to determine the extent and gender specificity of changes in the hypothalamic-pituitary-gonadal axis called experimental autoimmune encephalomyelitis in a rat multiple-sclerosis model. During the course of the disease, hypothalamic tissue showed transient upregulation of the inflammatory marker genes Gfap, Cd68, Ccl2, and Il1b in both sexes, but was accompanied by sex-specific decrease in Kiss1 (in females only) and Gnrh1 (in males only) expression. In females, the expression of the gonadotroph-specific genes Lhb, Cga, and Gnrhr was also inhibited, accompanied by decreased basal but not stimulated serum luteinizing hormone levels and temporary arrest of the estrous cycle. In contrast, Fshb expression and serum progesterone levels were temporarily elevated, findings consistent with the maintenance of the corpora lutea, and increased immunohistochemical labeling of ovarian StAR (steroidogenic acute regulatory protein), a protein that limits the rate of the steroidogenic pathway. In males, decreased regulation of Gnrhr expression and basal and stimulated serum LH and testosterone levels were accompanied by inhibited expression of the StAR protein in the testis. We suggest that inflammation of the hypothalamic tissue reduces the expression of Kiss1 and Gnrh1 in females and males, respectively, leading to sex-specific changes downstream of the axis [Reference 3].
Cell type–specific expression pattern of proton-sensing receptors and channels in pituitary gland
Extracellular protons act as orthosteric ligands for proton-sensing G protein–coupled receptors (GPRs) and acid-sensing ion channels (ASICs), as well as allosteric regulators of other GPRs and channels. Four orphan GPRs, GPR4, GPR65, GPR68, and GPR132, sense extracellular protons through histidine residues of the receptors and signal through heterotrimeric G-proteins, including Gs, Gi, Gq, and G12/13, triggering different intracellular signaling pathways. ASICs are a class of voltage-independent proton-gated sodium-conducting receptor channels. There are seven subunits (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, ASIC4, and ASIC5) encoded by five genes. All subunits are composed of two transmembrane domains, a large extracellular loop, and cytoplasmic N- and C-termini. Functional channels consist of three subunits in a homomeric or heteromeric configuration. The expression of these receptors and channels and their roles in pituitary-cell functions have not been systematically investigated.
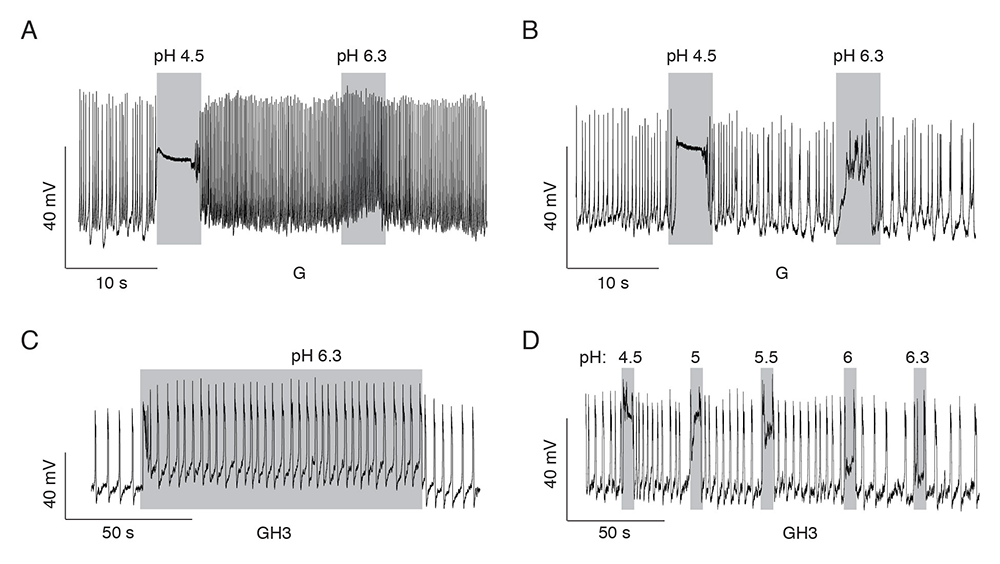
We recently completed a study on the cell type–specific expression pattern of proton-sensing GPRs and ASICs in rat anterior pituitary cells and GH3 immortalized lactosomatotrophs (GH3 cells). Our work includes scRNASeq, RT-PCR, and qRT–PCR analyses, double immunostaining, and single-cell electrophysiological analysis. qRT–PCR analysis revealed expression of the G protein–coupled receptor 68 gene (Gpr68) and the ASIC genes Asic1, Asic2, and Asic4 in anterior pituitary cells, and Asic1 and Asic2 in immortalized GH3 pituitary cells. Asic1a and Asic2b were the dominant splice isoforms. Single anterior pituitary cell RNA sequencing and immunocytochemical analysis showed that non-excitable folliculostellate cells express the GPR68 gene and protein, while excitable secretory cells express ASIC genes/proteins. Asic1 was detected in all secretory cell types, Asic2 in gonadotrophs, thyrotrophs, and somatotrophs, and Asic4 in lactotrophs only. Extracellular acidification activated two types of currents in a concentration-dependent manner: a rapidly developing, desensitizing current, with an estimated EC50 value of pH 6.7; and a slowly developing, non-desensitizing current, which required a higher proton concentration for activation. The desensitizing current was eliminated by removal of bath sodium and application of amiloride, a blocker of ASIC channels, whereas the non-desensitizing current was amiloride-insensitive and voltage-dependent. Activation of both currents increased the excitability of secretory pituitary cells, consistent with their potential physiological relevance in the control of voltage-gated calcium influx and calcium-dependent cellular functions [Reference 4].
Figure 2. Patterns of proton-induced changes in electrical activity in spontaneously firing single pituitary cells
A and B. Gonadotrophs expressing slow current (A) and both currents (B).
C and D. GH3 cells expressing both currents. A rapid high depolarization and a sustained low depolarization of sufficient amplitude to increase the frequency of firing of action potentials (C) and proton concentration dependence of the sustained low depolarization (D).
Expression and role of TSH receptors in proopiomelanocortin-producing pituitary cells
It is well established that thyroid-stimulating hormone (TSH) regulates thyroid hormone synthesis and release from the thyroid gland by activating TSH receptors expressed in thyroid follicular cells. In the human thyroid gland, TSH receptors not only interact with Gs and Gq/11 proteins (G protein coupled–receptors), leading to adenylyl cyclase and phospholipase C activation, respectively, but are also capable of signaling though Gi/o and G12/13 proteins [Reference 4]. Several extra-thyroidal expression sites of TSH receptors have also been reported [Reference 5], including in normal and adenomatous human pituitary tissue. However, the cell type(s) expressing the receptors have not been identified.
We recently examined whether the functional TSH receptors are also expressed in cultured rat pituitary cells, using double immunocytochemistry, qRT–PCR analysis, cAMP and hormone measurements, and single-cell calcium imaging. Double immunocytochemistry revealed the expression of TSH receptors in cultured corticotrophs and melanotrophs, in addition to previously identified receptors in folliculostellate cells. Functional coupling of the receptors with the Gq/11–signaling pathway was not observed, as evidenced by the lack of TSH activation of inositol trisphosphate (IP3)–dependent calcium mobilization in the cells when bathed in calcium-deficient medium. However, TSH increased cAMP production in a time- and concentration-dependent manner and facilitated calcium influx in single corticotrophs and melanotrophs, indicating their coupling to the Gs–signaling pathway. Consistent with these findings, TSH stimulated adrenocorticotropin and beta-endorphin release in male and female pituitary cells in a time- and concentration-dependent manner without affecting expression of the proopiomelanocortin gene. This ongoing research indicates that TSH is a potential paracrine modulator of anterior pituitary corticotrophs and melanotrophs, controlling the exocytotic but not the transcriptional pathway in a cAMP/calcium influx–dependent manner.
Publications
- Fletcher PA, Smiljanic K, Maso Prévide R, Iben JR, Li T, Rokic MB, Sherman A, Coon SL, Stojilkovic SS. Cell type- and sex-dependent transcriptome profiles of rat anterior pituitary cells. Front Endocrinol (Lausanne) 2019;10:623.
- Janjic MM, Previde RM, Fletcher PA, Sherman A, Smiljanic K, Abebe D, Bjelobaba I, Stojilkovic SS. Divergent expression patterns of pituitary gonadotropin subunit and GnRH receptor genes to continuous GnRH in vitro and in vivo. Sci Rep 2019;9:20098.
- Milosevic A, Janjic MM, Lavrinja I, Savic D, Bozic ID, Tesovic K, Jakovljevic M, Pekovic S, Stojilkovic SS, Bjelobaba I. The sex-specific patterns of changes in hypothalamic-pituitary-gonadal axis during experimental autoimmune encephalomyelitis. Brain Behav Immun 2020;89:233-244.
- Wang K, Kretschmannova K, Prévide RM, Smiljanic K, Chen Q, Fletcher PA, Sherman A, Stojilkovic SS. Cell type-specific expression pattern of proton sensing receptors and channels in pituitary gland. Biophys J 2020;119:1-14.
- Illes P, Müller CE, Jacobson KA, Grutter T, Nicke A, Fountain SJ, Kennedy C, Schmalzing G, Jarvis MF, Stojilkovic SS, King BF, Di Virgilio F. Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br J Pharmacol 2021;178(3):489-514.
Collaborators
- Ivana Bjelobaba, PhD, University of Belgrade, Beograd, Serbia
- Patrick A. Fletcher, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
- Arthur Sherman, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
Contact
For more information, email stojilks@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/stojilkovic or https://dir.ninds.nih.gov/Faculty/Profile/stanko-stojilkovic.html.