Hippocampal Interneurons and Their Role in the Control of Network Excitability

- Chris J. McBain, PhD, Head, Section on Cellular and Synaptic Physiology
- Ramesh Chittajulla, PhD, Staff Scientist
- Kenneth Pelkey, PhD, Staff Scientist
- James D'Amour, PhD, Postdoctoral Fellow
- June Hoan Kim, PhD, Postdoctoral Fellow
- Vivek Madahavan, PhD, Postdoctoral Fellow
- Geoffrey Vargish, PhD, Postdoctoral Fellow
- Steven Hunt, Biologist
- Xiaoqing Yuan, MSc, Biologist
- Tyler Ekins, BSc, Predoctoral Fellow
- Connie Mackenzie-Gray Scott, Bsc, Predoctoral Fellow
- Mandy Lai, BSc, Postbaccalaureate Fellow
- Areg Peltekian, BSc, Postbaccalaureate Fellow
Cortical and hippocampal GABAergic inhibitory interneurons (INs) are “tailor-made” to control cellular and network excitability by providing synaptic and extrasynaptic input to their downstream targets via GABAA and GABAB receptors. The axons of this diverse cell population make local, short-range projections (although some subpopulations project their axons over considerable distances) and release the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) onto a variety of targets. Distinct cohorts of INs regulate sub- and supra-threshold intrinsic conductances, regulate Na+- and Ca2+-dependent action-potential generation, modulate synaptic transmission and plasticity, and pace both local- and long-range large-scale synchronous oscillatory activity. An increasing appreciation of the roles played by INs in several neural-circuit disorders, such as epilepsy, stroke, Alzheimer’s disease, and schizophrenia, has seen this important cell type take center stage in cortical circuit research. With almost 30 years of interest in this cell type, the main objectives of the lab have been to understand: (1) the developmental trajectories taken by specific cohorts of INs as they populate the nascent hippocampus and cortex; (2) how ionic and synaptic mechanisms regulate the activity of both local circuit GABAergic INs and principal neurons (PN) at the level of small, well defined networks; and (3) how perturbations in their function alter the cortical network in several neural circuit disorders. To this end, we use a variety of electrophysiological, imaging, optogenetic, immunohistochemical, biochemical, molecular, and genetic approaches with both wild-type and transgenic animals.
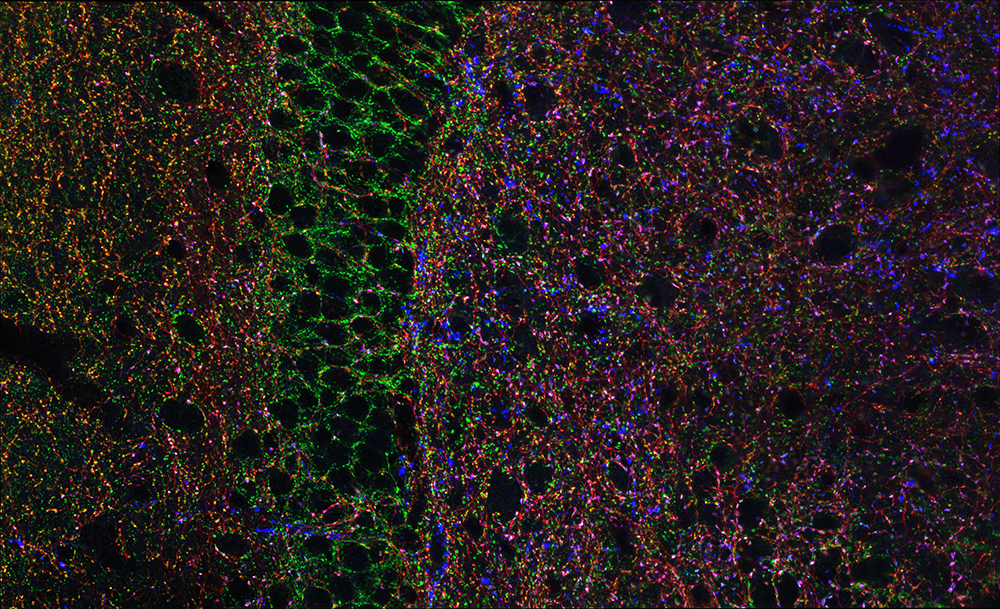
Figure 1. Hippocampal GABAergic terminals
Three major types of GABAergic terminal are shown in the hippocampal dentate gyrus (red, blue, and green punch). The black negative space is the cell bodies of principal cells innervated by the GABAergic terminals.
Paradoxical network excitation by glutamate release from VGluT3+ CCK–containing hippocampal GABAergic interneurons
Several neuronal subtypes utilize more than one classical neurotransmitter, in violation of Dale’s principle. Molecular identification of vesicular glutamate transporter 3– and cholecystokinin-expressing cortical interneurons (CCK+VGluT3+INTs) has prompted speculation of GABA/glutamate co-release from these cells for almost two decades despite a lack of direct evidence. We now have unequivocally demonstrated CCK+VGluT3+INT–mediated GABA/glutamate co-transmission onto principal cells in adult mice using paired whole-cell electrophysiological recording and optogenetic approaches. Although under normal conditions GABAergic inhibition dominates CCK+VGluT3+INT signaling, glutamatergic signaling becomes predominant when glutamate decarboxylase (GAD) function is compromised. CCK+VGluT3+INTs exhibit surprising anatomical diversity, comprising subsets of all known dendrite-targeting CCK+ interneurons in addition to the expected basket cells, and their extensive circuit innervation profoundly dampens circuit excitability under normal conditions. However, in contexts where the glutamatergic phenotype of CCK+VGluT3+INTs is amplified, they promote paradoxical network hyperexcitability, which may be relevant to disorders involving GAD dysfunction, such as schizophrenia or vitamin B6 deficiency. Such co-release is expected to impart the unique computational properties to CCK+VGluT3+INTS, differentiating them from CCKBCs lacking VGluT3, which are otherwise morphologically/electrophysiologically indistinguishable. Indeed, CCK+VGluT3+INTS exhibit unique participation in, and regulation of, hippocampal network oscillations and place-cell (cells that act as a cognitive representation of a specific location in space) spatial information coding.
Activity-dependent tuning of intrinsic excitability in mouse and human neurogliaform inhibitory interneurons
The ability to modulate the efficacy of synaptic communication between neurons constitutes an essential property critical for normal brain function. Animal models have proved invaluable in revealing a wealth of diverse cellular mechanisms underlying varied plasticity modes. However, to what extent these processes are mirrored in humans is largely uncharted, thus questioning their relevance to human circuit function. In this study, led by Ramesh Chittajallu, we focus on a novel type of inhibitory interneuron, the neurogliaform cells (NGFC), which possess specialized physiological features enabling them to impart a widespread inhibitory influence on neural activity. We demonstrated that this prominent neuronal subtype, embedded in both mouse and human neural circuits, undergoes remarkably similar activity-dependent modulation, manifesting as epochs of enhanced intrinsic excitability. In principle, these evolutionary conserved plasticity routes likely tune the extent of neurogliaform cell–mediated inhibition, thus constituting canonical circuit mechanisms underlying human cognitive processing and behavior.
Comparative studies such as the one described here are vital for determining to what extent circuit features gleaned from experimental animal models are relevant in humans. Particularly, we uncovered a previously undescribed evolutionarily conserved mechanism that manifests as a short-term enhancement in the propensity of depolarizing inputs to evoke action potential output. Remarkably, amongst the varied IN located in superficial regions of cortical and hippocampal microcircuits, NGFCs in both species were found to be privileged with regard expression of these forms of plasticity. Together, our data reveal the presence of cellular mechanisms that result in modulation of intrinsic excitability of NGFCs that represent circuit motifs important for human brain function.
Translatome analysis using conditional ribosomal tagging in GABAergic interneurons and other sparse cell types
GABAergic interneurons comprise a small but diverse subset of neurons in the mammalian brain, which tightly regulate neuronal circuit maturation and information flow and, ultimately, behavior. Because of their centrality in the etiology of numerous neurological disorders, examining the molecular architecture of these neurons under various physiological scenarios has piqued the interest of the broader neuroscience community. The last few years have seen an explosion in next-generation sequencing (NGS) approaches aimed at identifying genetic and state-dependent subtypes in neuronal diversity. Although several approaches are employed to address neuronal molecular diversity, ribosomal tagging has emerged at the forefront of identifying the translatomes of neuronal subtypes, an approach that primarily relies on Cre recombinase-driven expression of hemagglutinin A (HA)-tagged RiboTag mice exclusively in the neuronal subtype of interest. This allows the immunoprecipitation of cell type–specific, ribosome-engaged mRNA, expressed both in the soma and the neuronal processes, for targeted quantitative real-time PCR (qRT-PCR) or high-throughput RNA sequencing analyses. Vivek Mahadevan and Areg Peltekian described the typical technical caveats associated with successful application of the RiboTag technique for analyzing GABAergic interneurons, and in theory other sparse cell types, in the central nervous system.
AMPA receptor deletion in developing MGE–derived hippocampal interneurons causes a redistribution of excitatory synapses and attenuates postnatal network oscillatory activity.
Inhibitory interneurons derived from the medial ganglionic eminence (MGE) represent the largest cohort of GABAergic neurons in the hippocampus. In the CA1 hippocampus, excitatory synapses onto these cells comprise GluA2–lacking, calcium-permeable AMPA receptors (AMPARs). Although synaptic transmission is not established until early in their postnatal life, AMPARs are expressed early in development; their role is however enigmatic. Former postdoctoral fellow Gülcan Akgül genetically deleted GluA1, GluA2, and GluA3 selectively from MGE–derived interneurons early in development, using the Nkx2.1-cre mouse line. We observed that the number of MGE–derived interneurons was preserved in the mature hippocampus despite early elimination of AMPARs, which resulted in an over 90% reduction in spontaneous excitatory synaptic activity. Of particular interest is the observation that excitatory synaptic sites were shifted from dendritic to somatic locations while maintaining a normal NMDAR content. The developmental switch of NMDARs from GluN2B–containing early in development to GluN2A–containing on maturation was similarly unperturbed despite the loss of AMPARs. The oscillatory activity of early network-driven giant depolarizing potentials was compromised in early postnatal days, as were both feedforward and feedback inhibition onto pyramidal neurons, underscoring the importance of glutamatergic drive onto MGE–derived interneurons for hippocampal circuit function.
Additional Funding
- Jason Wester was funded by an NINDS Intramural National Research Service Award (NRSA).
- James D’Amour was funded by an NIGMS PRAT Fellowship.
Publications
- Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, McBain CJ. Hippocampal GABAergic inhibitory interneurons. Physiol Rev 2017;97:1619-1747.
- Akgul G, Abebe D, Yuan X-Q, Auville K, McBain CJ. AMPA receptor deletion in developing MGE-derived hippocampal interneurons causes a redistribution of excitatory synapses and attenuates postnatal network oscillatory activity. Sci Rep 2020;10:1333.
- Pelkey KA, Calvigioni D, Fang C, Vargish G, Ekins T, Auville K, Wester JC, Lai M, Mackenzie-Gray Scott C, Yuan X, Hunt S, Abebe D, Xu Q, Dimidschstein J, Fishell G, Chittajallu R, McBain CJ. Paradoxical network driven excitation by glutamate release from VGluT3+ GABAergic interneurons. eLife 2020;9:e51996.
- Mahadevan V, Peltekian A, McBain CJ. Translatome analyses using ribosomal tagging in GABAergic interneurons and other sparse cell types. Curr Protoc Neurosci 2020;92:e93.
- Chittajallu R, Auville K, Mahadevan V, Lai M, Hunt S, Pelkey KA, Zaghloul K, McBain CJ. Activity dependent tuning of intrinsic excitability in mouse and human neurogliaform cells. eLife 2020;9:e57571.
Collaborators
- Jordan Dimidschstein, PhD, The Broad Institute, Cambridge, MA
- Gordon Fishell, PhD, The Broad Institute, Cambridge, MA
- Timothy J. Petros, PhD, Unit on Cellular and Molecular Neurodevelopment, NICHD, Bethesda, MD
- Kareem Zaghloul, MD, PhD, Functional and Restorative Neurosurgery Unit, NINDS, Bethesda MD
Contact
For more information, email mcbainc@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/mcbain or https://dir.ninds.nih.gov/Faculty/Profile/chris-mcbain.html.