Structural and Chemical Biology of Membrane Proteins

- Anirban Banerjee, PhD, Head, Unit on Structural and Chemical Biology of Membrane Proteins
- Natalia Gomez Navarro, PhD, Research Fellow
- Elliot Murphy, PhD, Postdoctoral Intramural Research Training Award Fellow
- Robbins Puthenveetil, PhD, Postdoctoral Intramural Research Training Award Fellow
- Tanmay Mondal, PhD, Visiting Fellow
- Elvis Ongey Legala, PhD, Visiting Fellow
- Rahul Raina, PhD, Visiting Fellow
- Kevin Choi, BS, Postbaccalaureate Fellow
- Sarah Miller, BS, Postbaccalaureate Fellow
Molecular mechanism of post-translational protein lipidation by zDHHC protein S-acyltransferases
Post-translational modifications greatly expand the structural, chemical, and functional diversity of the proteome. Of these, protein lipidation, which collectively refers to covalent modification of proteins by lipids, constitutes a centrally important class of post-translational modification. Protein S-acylation, commonly known as protein palmitoylation, is a specific form of protein lipidation whereby long-chain fatty acids, typically C16, become covalently attached to cytosol-facing cysteines through a thioester linkage. Palmitoylation is one of the most pervasive and physiologically important post-translational modifications, and the targets of palmitoylation span a very wide range of proteins, ranging from ion channels to cell-surface receptors, neuronal scaffolding proteins, and small GTPases. The repertoire of palmitoylated proteins has expanded rapidly in recent years, with thousands of proteins now known to be part of the cellular ‘palmitoylome.’ The physico-chemical effect of palmitoylation is to alter the local hydrophobicity of the substrate protein. The thioester bond makes S-acylation unique in that it is a labile moiety and can be cleaved, in the cellular context, by thioesterase enzymes, which makes S-acylation one of the few dynamic post-translational modifications and unique among different forms of protein lipidation. The physiological effects of S-acylation are diverse and have critical cellular importance. For example, Ras, a small GTPase that is critical for cellular growth and differentiation and is mutated in about one-third of all human cancers, is palmitoylated at the Golgi and subsequently targeted to the plasma membrane by vesicular transport. Palmitoylated Ras localizes to cholesterol-rich domains on the plasma membrane. However, it is subsequently depalmitoylated by the thioesterase APT1, dissociates from the plasma membrane, and redistributes on endomembranes, including the Golgi. Such dynamic recycling of Ras is critical for its function. On the other hand, in recent work (see below), we showed that the Spike protein of SARS-CoV-2, the causative agent of COVID-19, is S-acylated, which is important in the viral life cycle.
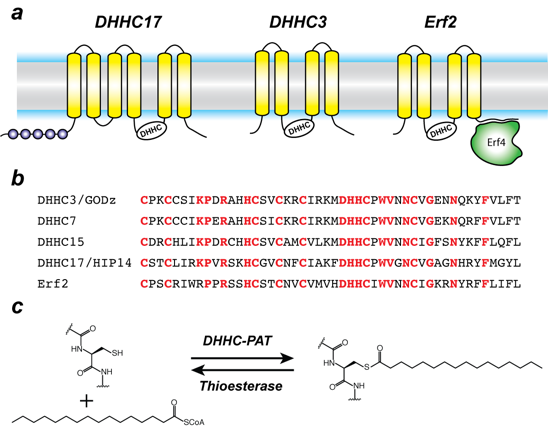
Protein S-acylation is catalyzed by a large group of enzymes known as zDHHC palmitoyl acyltransferases (also referred to as DHHC enzymes or DHHC-PAT), so named because they contain a signature D-H-H-C motif (aspartate-histidine-histidine-cysteine) in a cysteine-rich domain (CRD) in an intracellular loop (Figure 1). These are low-abundance polytopic integral membrane proteins localized to a variety of cellular compartments. Humans have 23 DHHC-PATs encoded in their genome. Beyond the shared DHHC domain, DHHC-PATs vary considerably; some possess ankyrin repeats (structural protein motifs that mediate protein-protein interactions), a few have six transmembrane helices instead of the usual four, and at least one forms a functional heterodimer with a cytoplasmic auxiliary subunit. To date, no consensus sequences have been reported for palmitoylation. A specific DHHC-PAT can palmitoylate many substrates, and, conversely, a given substrate can be palmitoylated by many DHHC-PATs. Such redundancy has been one of the most intriguing aspects of DHHC-PATs and makes it difficult to assign substrates by overexpression/knockout strategies, given that, in the absence of one DHHC-PAT enzyme, others can take over. However, this does not necessarily reflect the true enzyme-substrate relationship. The situation is even more confounded by the lack of specific inhibitors of DHHC-PATs. Even though 2-bromopalmitate is widely used as a global inhibitor of DHHC-PATs, it has been shown that it also broadly targets other proteins involved in lipid metabolism.
Figure 1. Organization and properties of DHHC palmitoyltransferases (PATs)
a. The organization of three different DHHC-PATs are shown schematically. The spheres indicate protein-protein interaction domains. Erf2 associates with a cytoplasmic subunit, Erf4, to form the active enzyme.
b. The DHHC-CRD region of a few representative DHHC-PATs are aligned. The conserved amino acids are shown in red.
c. Reaction catalyzed by DHHC-PATs; the reverse reaction is catalyzed by acylprotein thioesterases (APT).
Besides its broad importance in cell biology, palmitoylation has been linked to several diseases, most notably neuro-psychiatric disorders such as Huntington’s disease and various forms of cancer. Recently, it was shown that zDHHC20 palmitoylates epidermal growth factor (EGFR) and is thus a potential therapeutic target for a wide range of cancers. More recently, zDHHC3 has been proposed as a target for cancer treatment owing to its activity as the palmitoyltransferase for the programmed cell-death ligand 1 (PD-L1). However, when we started working on this family, very little was known about the molecular mechanism of zDHHC palmitoyltransferases, despite their importance across a broad spectrum of biological pathways and their biomedical importance. Nothing was known about their structural organization or how they interact with substrates and the fatty acyl coenzyme A (CoA), which serves as the acyl donor.
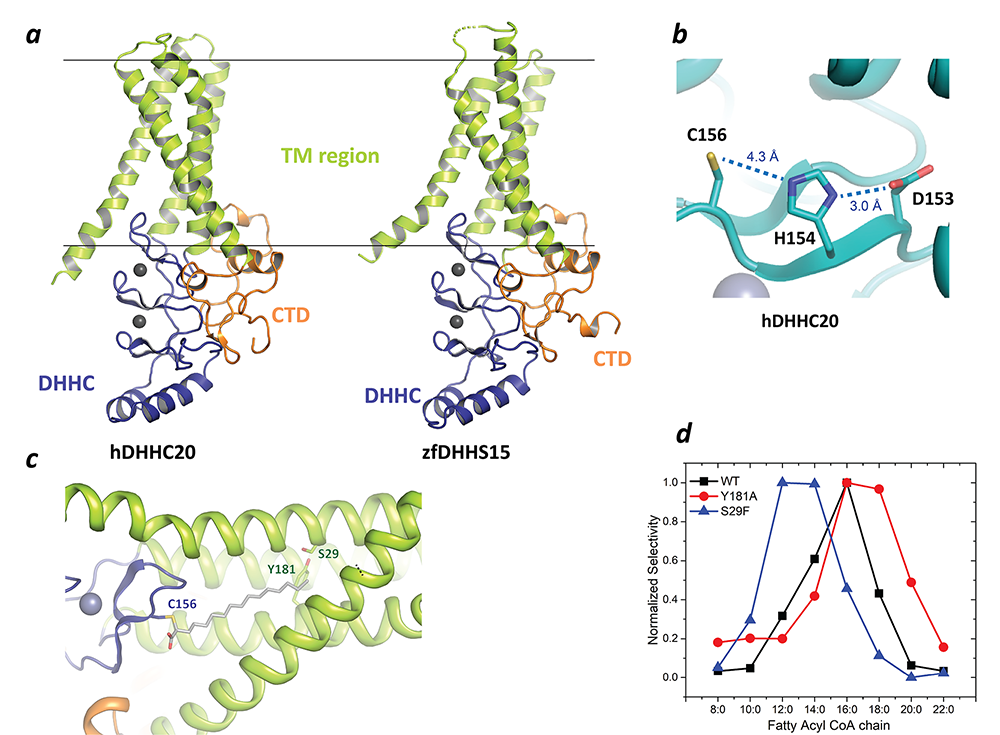
In a major breakthrough in this field, we had earlier solved the high-resolution crystal structures of two members of the zDHHC family, human zDHHC20 (hDHHC20) and zebrafish zDHHC15 (Figure 2a), the first structures of any member of this family to be characterized. They reveal a tepee-like transmembrane domain organization, which splays apart towards the cytoplasmic side and harbors the active site at the membrane-aqueous interfacial region (Figure 2b), thus readily explaining why membrane-proximal cysteines are palmitoylated. We also solved the structure of hDHHC20 irreversibly modified by a covalent inhibitor, 2-bromopalmitate. The structure mimics the auto-acylated intermediate state in the enzymatic pathway and thus reveals how the acyl group of fatty acyl-CoA binds in a cavity formed in the bilayer by the transmembrane domain (Figure 2c). Residues lining the cavity contact the acyl chain, and their mutation affects enzymatic activity. By mutating two residues at the tapering end of the cavity, we also showed that we can change the acyl chain–length selectivity of the mutant enzymes (Figure 2d). Thus, the cavity functions as a molecular ruler in determining the acyl chain–length selectivity of hDHHC20, important because, although palmitate is the most prevalent fatty acid used by DHHC palmitoyltransferases, they can use fatty acyl–CoAs of other chain lengths, a property that varies between different members of the DHHC family.
Figure 2. Structure, function, and membrane deformation of DHHC palmitoyltransferases
a. The structure of human DHHC20 and a catalytically inactive mutant of zebrafish DHHC15 shown in ribbon trace: the transmembrane domain (TM) is shown in green, the DHHC–containing cysteine-rich domain in blue and the C-terminal domain in orange; the grey spheres indicate Zn2+ ions; these are both Golgi-resident enzymes, and thus the top side faces the Golgi lumen and the active site the cytoplasm.
b. Active site of human DHHC20 showing the catalytic triad containing the active-site cysteine.
c. Structure of human DHHC20 irreversibly modified with 2-bromopalmitate, which results in the active-site cysteine linking to the alpha-carbon of palmitic acid: the acyl group of palmitic acid is shown in stick rendition; also shown are two residues towards the top of the tapering cavity to where the palmitate binds.
d. The acyl chain–length selectivity of wild-type (WT) human DHHC20. Mutation of tyrosine181 to alanine (Y181A) expands the cavity and shifts the acyl selectivity to the longer side. On the other hand, mutation of serine29 to phenylalanine (S29F) contracts the cavity and thus shifts the acyl selectivity to the shorter side.
Our goal is to obtain a structural snapshot of every state in the catalytic pathway of zDHHC enzymes. To this end, we recently used a combination of x-ray crystallography, atomistic molecular dynamics simulations, and biochemistry to obtain and validate a structure of the pre-catalytic complex of human zDHHC20 with palmitoyl CoA. The structure (Figure 3a) reveals that, while the hydrophobic fatty acyl chain is recognized by the transmembrane cavity of zDHHC20, the polar head group of palmitoyl CoA is recognized by the cytoplasmic charge-complementary surface. The functional and computational data show that free CoA does not compete effectively with palmitoyl CoA, leading to the hypothesis that, in this context, long chain fatty acyl CoAs act as bivalent ligands, with a hydrophobic chain and the polar head group linked by a flexible linker (Figure 3b). Coincident recognition of both modules by protein palmitoyltransferases (Figure 3b) is necessary for catalytic chemistry to take place.
Figure 3. Bivalent recognition of palmitoyl CoA by human zDJHC20
a. Structure of the precatalytic complex of a catalytically inactive mutant of human zDHHC20 with palmitoyl CoA: the protein is shown in surface rendition colored by electrostatic potential with positively charged surface in blue and negative charged surface in red; the palmitoyl CoA is shown in ball and stick rendition with natural atom colorings except carbon, which is shown as white spheres; the two lines indicate approximate bilayer boundaries.
b. Schematic showing bivalent recognition of long chain fatty acyl CoA by palmitoyltransferases: the chemical structure of palmitoyl CoA is also shown with the fatty acyl chain, CoA head group, and the pantetheine linker indicated.
S-acylation was first discovered in viral proteins and, in the wake of the COVID-19 pandemic, we turned our attention to this. It had been shown that the Spike protein of other coronaviruses or its equivalent in other pathogenic viruses are S-acylated and, in a subset of these, S-acylation is crucial to the viral life cycle. However, it was not known whether the Spike protein of SARS-CoV-2, the causative agent of COVID-19, is S-acylated and, if so, how that affects the viral life cycle. In previous work, we demonstrated that SARS-CoV-2 Spike is S-acylated and, in collaboration with Eric Freed's lab, showed that S-acylation is critically important for the viral life cycle. Remarkably, even 40 years after the discovery of S-acylation, there were still no in vitro reconstitution of S-acylation of any viral protein with purified components. We reconstituted the S-acylation of SARS-CoV-2 Spike protein in vitro, using purified zDHHC enzymes, and observed a striking correlation with our in cellulo experiments. The study also opened up avenues for further mechanistic dissection and laid the groundwork for identifying targeted small-molecule modulators. Recently, we expanded our focus to the S-acylation of Influenza HA protein and HIV-1 Tat protein. Both had been shown to be S-acylated, but the mechanistic underpinnings remain unclear. We are developing assays for in vitro reconstitution of the S-acylation of Influenza HA and HIV-1 Tat. These assays will provide the ground work for dissecting the mechanism whereby zDHHC enzymes recognize these substrates and catalyze S-acylation.
Molecular mechanism of iron and polyamine transport across cellular membranes
The importance of iron in biology cannot be overstated. In higher organisms, mitochondria are the ‘hotspot’ for the cell biology of iron, because Fe-S clusters are biosynthesized and iron is inserted into heme there. Mitochondrial iron homeostasis plays a critical role in cellular iron homeostasis and in the overall physiology of the cell. In vertebrates, the only known major transporters of iron into mitochondria are mitoferrin-1 and mitoferrin-2, two homologous members of a large group of mitochondrial transporters known as the Mitochondrial Carrier family (Figure 4). Mitoferrin-1 (Mfrn1) is expressed mainly in erythroid cells, while mitoferrin-2 is expressed ubiquitously. Knockout of Mfrn1 is embryonically lethal, reflecting the importance of mitoferrins in vertebrate physiology.
Mfrn1 and Mfrn2 were discovered more than 15 years ago. However, the proposed iron-transport activity had not been demonstrated using an in vitro functional reconstitution assay, and nothing was known about their interaction with iron or other related metal ions, most likely because heterologous overexpression and purification of mitoferrins were not reported in the literature. There were also no reports of a reconstituted iron transport assay starting from purified protein. In earlier work, we carried out heterologous purification, in vitro functional reconstitution, and mutational dissection of a vertebrate Mfrn1 (Figure 4), the first demonstration that Mfrn1 can indeed transport iron. Our studies provided the first biochemical insights into Mfrn function. In earlier work, we also used our in vitro proteoliposome-reconstituted iron-transport assay, the first such assay to be reported in the literature, to dissect the iron-transport activity of MavN, another highly conserved iron transporter, in the bacterial pathogen Legionella pneumophila (Figure 4b).
In the past two years, we shifted our interest to transporters that move polyamines across the membrane. Polyamines, small organic polycations, are essential for cell viability, and their physiological levels are homeostatically maintained by post-transcriptional regulation of key biosynthetic enzymes. In addition to de novo synthesis, cells can also take up polyamines; however, until recently, dedicated polyamine transporters were not known. Tom Dever's lab discovered that the S. cerevisiae HOL1 mRNA is under translational control by polyamines. They also showed that Hol1 is required for yeast growth under limiting polyamine conditions. These experiments suggested that Hol1 is a high-affinity polyamine transporter (Figure 4c). We purified recombinant Hol1 and reconstituted it in proteoliposomes to demonstrate that it indeed shows robust polyamine transport activity. An extensive set of experiments from the Dever lab bolstered the conclusions that Hol1 is a high-affinity polyamine transporter and under translational autoregulation by polyamines in yeast, highlighting the extensive control cells impose on polyamine levels. We are now investigating the underlying molecular mechanisms behind Hol1 function.
Figure 4. Transporters of iron and polyamine
a. Iron is imported through the plasma membrane by the transferrin/transferrin receptor (blue) cycle and is transported out of endosomes by the divalent metal ion transporter (DMT) (grey); iron is delivered to mitoferrin (yellow cylinders) by unknown means; mitoferrin delivers iron to unknown partners in mitochondria, which become available for heme and Fe-S cluster biosynthesis.
b. Schematic showing Legionella entering a host cell and sequestering itself in a Legionella-containing vacuole (LCV): MavN is inserted into the membrane of the LCV and hijacks iron from the host cell.
c. Assay showing iron transport by proteoliposome-reconstituted MavN: Hol1 as the major high-affinity polyamine transporter in S. cerevisiae; polyamines autoregulate HOL1 expression through feedback inhibition of HOL1 mRNA translation, mediated by polyamine inhibition of eIF5A–dependent translation termination on a conserved upstream open reading frame in the HOL1 mRNA leader.
d. SDS-PAGE gel of detergent-purified K. lactis Hol1 (arrowhead).
e. [14C]Spermidine uptake by control or K. lactis Hol1 proteoliposomes; error bars denote SD, **p < 0.01 (Student’s two-tailed t test, n = 3).
Additional Funding
- Office of AIDS Research
Publications
- Lee CJ, Stix R, Rana MS, Shikwana F, Murphy RE, Ghirlando R, Faraldo-Gómez JD, Banerjee A. Bivalent recognition of fatty acyl-CoA by a human integral membrane palmitoyltransferase. Proc Natl Acad Sci USA 2022 119 (7):e2022050119.
- Puthenveetil R, Gomez-Navarro N, Banerjee A. Access and utilization of long chain fatty acyl-CoA by zDHHC protein acyltransferases. Curr Opin Struct Biol 2022 77:102463.
- Murphy RE, Banerjee A. In vitro reconstitution of substrate S-acylation by the zDHHC family of protein acyltransferases. Open Biology 2022 12:210390.
- Puthenveetil R, Lun CM, Murphy RE, Healy LB, Vilmen G, Christenson ET, Freed EO, Banerjee A. S-acylation of SARS-CoV-2 spike protein: mechanistic dissection, in vitro reconstitution and role in viral infectivity. J Biol Chem 2021 297(4):101112–101124.
- Vindu A, Shin BS, Choi K, Christenson ET, Ivanov IP, Cao C, Banerjee A, Dever TE. Translational autoregulation of the S. cerevisiae high-affinity polyamine transporter Hol1. Mol Cell 2021 81(19):3904–3918.
Collaborators
- David Cafiso, PhD, Department of Chemistry, University of Virginia, Charlottesville, VA
- Thomas Dever, PhD, Section on Mechanism and Regulation of Eukaryotic Protein Synthesis, NICHD, Bethesda, MD
- José Faraldo-Gómez, PhD, Theoretical Molecular Biophysics Section, NHLBI, Bethesda, MD
- Eric Freed, PhD, Retroviral Replication Laboratory, NCI, Frederick, MD
- Rodolfo Ghirlando, PhD, Laboratory of Molecular Biology, NIDDK, Bethesda, MD
- James Inglese, PhD, Assay Development & Screening Technology Laboratory, NCATS, Bethesda, MD
- Ralph Isberg, PhD, Tufts University School of Medicine, Boston, MA
- Anant Menon, PhD, Weill Cornell Medicine, New York, NY
Contact
For more information, email anirban.banerjee@nih.gov or visit https://banerjee.nichd.nih.gov.