Point of Care and Wearable Biophotonics for Characterizing Tissue Composition and Metabolism

- Bruce Tromberg, PhD, Head, Section on Biomedical Optics, Director of the National Institute of Biomedical Imaging and Bioengineering
- Timothy Quang, PhD, Staff Scientist
- Brian Hill, MS, Biomedical Engineer
- Yun He, PhD, Postdoctoral Fellow
- Elise Berning, BS, Postbaccalaureate Fellow
- Sully Chen, BS, Postbaccalaureate Fellow
- Careniena Opem, BS, Postbaccalaureate Fellow
- Eric Gallagher, Student Fellow
- Kathryn Jaroszynski, Student Fellow
By advancing models, methods, and devices that utilize the interaction of light with biological tissue, we strive to develop non-invasive techniques that can help guide therapy and aid in clinical decision making. The techniques are used to perform real-time quantitative measurements of clinically relevant information, including tissue blood flow, oxygen extraction, and body/tissue composition. Our research seeks to move such technologies from ‘bench to bedside,’ where they can be applied to clinical problems, including vascular and metabolic diseases.
Optical characterization of vascular health in sickle cell disease
Sickle cell disease (SCD) is an inherited hemoglobinopathy that disproportionately impacts minority populations in the United States and affects about 100,000 individuals. The substitution of valine by glutamic acid on the 6th amino acid of beta globin results in an abnormal hemoglobin variant (HbS), which polymerizes once deoxygenated and alters both the structure and function of red blood cells (RBCs), distorting them into a ‘sickle’ shape. Sickled RBCs have ⅙th of the lifespan of normal RBCs, resulting in chronic hemolytic anemia; they are also rigid and can obstruct microvascular blood flow, causing recurring, unpredictable cycles of acute vaso-occlusive pain, commonly referred to as vaso-occlusive crises (VOC). Recurrent VOCs lead to hypoxia-reperfusion injury and, together with the intravascular hemolysis, promote inflammation and vascular endothelial damage. The systemic vasculopathy affects many organs, leading to cardiovascular complications, chronic pain, and cerebral and kidney impairment. Given that current treatment options are not universally effective, there is a significant, unmet need for new technologies that can quantitatively characterize SCD physiology and provide new insights for optimizing therapeutic impact in SCD patients.
Given the impairments to microvascular flow and endothelial dysfunction associated with SCD, advanced quantitative NIRS (near-infrared spectroscopy) is an attractive candidate to provide comprehensive hemodynamic evaluations in point-of-care settings. We are optically characterizing tissue composition, metabolism, and perfusion in several SCD studies, led by our collaborators Swee Lay Thein, Arun Shet, and Courtney Fitzhugh:
- NCT04610866 – Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Long-term Mitapivat Dosing in Subjects With Stable Sickle Cell Disease: An Extension of a Phase I Pilot Study of Mitapivat
- NCT05604547 – Exploring Near Infrared Spectroscopy (NIRS) Technologies for Assessment of Muscle Physiology, Tissue Oxygenation, and Blood Flow in Patients With Sickle Cell Disease (SCD)
- NCT04514510 – Fixed Dose Flavonoid Isoquercetin on Thrombo-Inflammatory Biomarkers in Subjects With Stable Sickle Cell Disease
- NCT05213572 – Observational Study to Deeply Phenotype Major Organs in Sickle Cell Disease After Curative Therapies
Our group is assessing the sensitivity of various optical devices to hemodynamic changes induced by SCD treatments and evaluating whether the changes correlate with what is observed from blood chemistry. Our latest preliminary findings are noted in Figures 1 and 2.
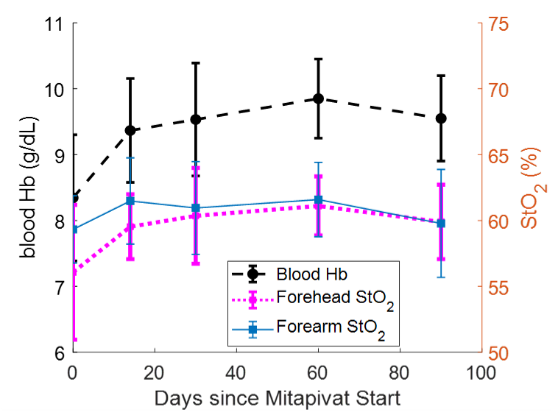
Figure 1. Average brain (pink) and forearm (blue) StO2 values compared with average blood Hb level over the first three months of a Mitapivat study (n = 9 pts)
Comparison of the mean blood hemoglobin (Hb) level acquired from blood draws to the mean forehead StO2 and mean forearm StO2, measured by time-domain near-infrared spectroscopy (TD-NIRS) across the first three months of treatment. We calculated optical hemodynamic metrics such as mean resting baseline concentrations of [O2Hb], [HHb], and [THb] as well as tissue oxygen saturation (StO2) in the forearm and forehead for all 45 measurement visits; one visit was excluded as the result of poor data quality. On average, forehead StO2 acquired by TD-NIRS trended similarly to the Hb level acquired by blood draws over the first three months. We observed that 7 of 9 patients exhibited a sustained increase in blood Hb compared with baseline; the mean Hb increase for all patients compared with baseline was 1.2 g/dL at the end of three months. Similarly, forehead StO2 also showed a similar, sustained increase. The mean forehead StO2 increase after three months over baseline was 4.0%.
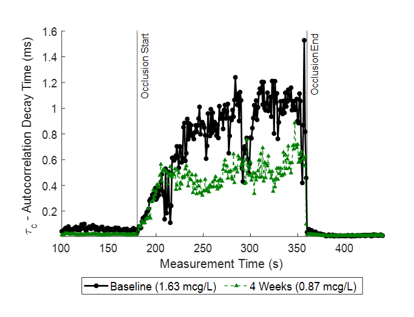
Figure 2. Change in autocorrelation decay time during brachial cuff occlusion during baseline and four-week follow-up visits for a single Isoquercetin patient; D-dimer levels are noted in the legend.
Change in autocorrelation decay time (τ) during the entire occlusion measurement at the baseline (black) and four-week follow-up (green) visits for one patient. The decay time τ is recovered using diffuse correlation spectroscopy (DCS) and reflects the dynamic properties of the blood. We can observe a reduction in τ (1.01 ms vs 0.57 ms), which corresponds with a reduction in D-dimer levels from 1.63 µg/mL to 0.87 µg/mL. Additionally, we observe that τ plateaus faster at the follow-up visit than at the baseline visit.
In this preliminary analysis of 10 patients with baseline and follow-up visits, we found agreement between the mean τ and slope to D-dimer levels. Three patients showed opposing trends in τ and d-dimer levels and one patient showed a reduction in τ and no change in d-dimer levels. Further analysis will evaluate whether measures of vascular reactivity correlate with biomarker of endothelial activity such as VCAM-1 (vascular cell adhesion protein-1).
Development of a wearable point-of-care monitoring device for pediatric obstructive sleep apnea
Obstructive sleep apnea (OSA) is the most common type of sleep apnea, in which the blockage of the airway causes the patient to stop breathing involuntarily for ten seconds or more throughout the night during sleep. When breathing stops, the oxygen level in the blood falls, sometimes waking the sleeper. Pediatric obstructive sleep apnea (POSA) can be particularly concerning, with several associated morbidities, which can have long-term effects extending into adulthood, including adverse changes in cardiovascular, metabolic, and developmental health. Onset usually occurs between ages 2–8, as tonsils reach their peak growth. Unfortunately, pediatric OSA remains largely under-diagnosed because of a lack of education about symptoms and the limited availability of sleep-medicine physicians. Early diagnosis and treatment are imperative for preventing many of these morbidities.
Additionally, the mechanisms of OSA that explain OSA–related outcome measures involving brain health and degeneration are still largely unknown. Approximately 50% of patients with OSA are excessively sleepy during the day, and many develop cardiovascular disease, cerebrovascular disease, and/or cognitive impairment, particularly if untreated. Traditional OSA measures do not predict sleepiness, and the apnea-hypopnea index (AHI) and oxygen desaturation index (ODI), measured through standard pulse oximetry, do not typically explain health outcomes, although both hypoxemia and sleep fragmentation are mildly associated.
NIRS is a non-invasive technology that is well suited for measuring cerebral hemodynamics during sleep. NIRS uses near-infrared light to penetrate human tissue and measure changes in oxygenation. There is extensive literature documenting the utility of NIRS in sleep studies of both normal and sleep-disordered breathing (SDB). In normal sleep, NIRS has been used to investigate cerebral hemodynamics in sleep stages and transitions between stages. For sleep apnea applications, NIRS can detect drops in cerebral oxygenation, as well as respiration, heart rate, and blood flow changes that occur due to apnea. Previous work in NIRS sleep analysis showed that transient rises in cerebral deoxyhemoglobin are prominent feature of apneas and hypopneas, and autoregulatory mechanisms have been shown to fail in preventing hypoxia during severe obstructive events. NIRS is uniquely suited to capture such breathing-induced cerebrovascular disturbances with high sensitivity and temporal resolution.
NCT05052216 – Development of a Wearable Point of Care Monitoring Device for Pediatric Obstructive Sleep Apnea
In collaboration with Ashura Buckley and the NIMH Sleep and Neurodevelopment Service (SNS), we are currently enrolling children aged 3–8 to investigate how optical signals change throughout sleep by comparing physiological signals derived from NIRS with data from polysomnography. Tissue hemodynamics of a healthy child during a pause in breathing are shown in Figure 3.
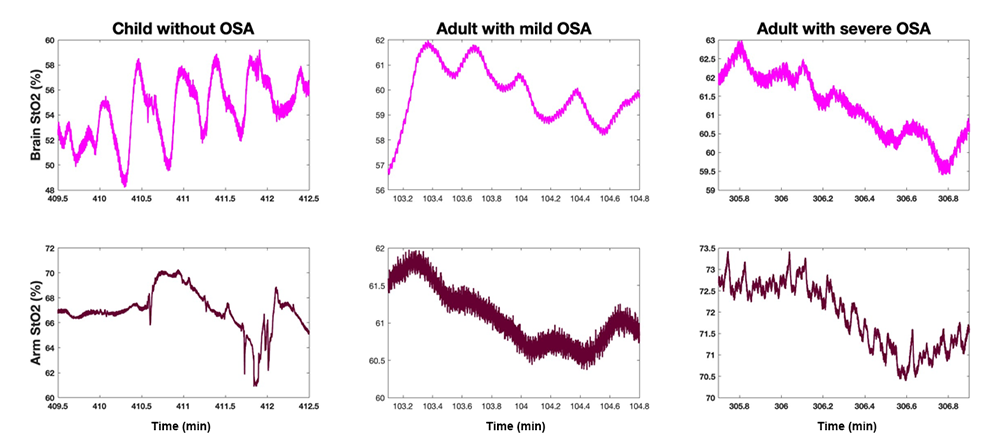
Figure 3. Change in arm and cerebral tissue oxygenation during a breathing stoppage for a healthy child, adult with mild OSA, and adult with severe OSA
Comparison of hemodynamic response during sleep to a breathing stoppage for a healthy child, adult with mild OSA, and adult with severe OSA. While response in the arm is similar across the three cases, with tissue oxygenation dropping shortly after the pause in breathing, there is a stark difference in the cerebral hemodynamic response. While adults with OSA also see a drop in cerebral oxygen saturation following the breathing stoppage, no net reduction is seen in the healthy child. The results suggest that OSA leads to impaired autoregulatory mechanisms, mechanisms that serve to protect the brain from hypoxia.
Vascular diseases driven by genetic alterations and COVID infection
The study and management of diseases with unknown vascular phenotypes is challenging. Given the importance of the vascular network to every organ system, understanding the extent and severity of vascular complications is necessary in order to develop effective treatments. Monogenic vascular diseases are characterized by a single genetic mutation, which can have deleterious effects on protein structure, function, or synthesis, and can be associated with severe complications, with high mortality and morbidity rates. While each individual disease is rare, these diseases can encompass a wide array of vascular phenotypes affecting any part of the body. Infectious diseases are another pathway for vascular complications, which can either manifest directly by viral/bacterial infection of the endothelial lining or indirectly as a result of damage triggered by inflammatory responses to the pathogen.
For this project, we aim to develop multi-modal NIRS assessment methods that are sensitive to the hemodynamic impairments of two patient cohorts: (1) patients with rare monogenic vascular disease; and (2) patients recovering from COVID-19. This consists of the selection of both the appropriate optical modality and measurement challenge to characterize the afflicted area. Our goal is to perform comprehensive clinical evaluations of patients recruited into the study, in order to better understand the disease pathology, heterogeneity of symptoms within the disease population, and progression of the various, rare vascular diseases over time. In collaboration with the Translational Vascular Medicine Branch (TVMB) at the NHLBI, three specific disease cohorts will be explored: ACDC (arterial calcification due to CD73 deficiency), CADASIL (cerebral autosomal dominant arteriopathy and leukoencephalopathy), and COVID-19.
NCT03538639 – Vascular Disease Discovery Protocol
ACDC is characterized by progressive vascular calcification, typically affecting arteries of the lower limbs, and manifests clinically as debilitating lower extremity pain resulting from the lower limb claudication and ischemia. For the ACDC cohort, we will evaluate vascular reactivity and endothelial function in the lower limbs using a hyperthermia challenge with an optical probe combining diffuse reflectance spectroscopy (DRS) and laser doppler flowmetry (LDF) (Perimed AB, Sweden) to evaluate vascular reactivity in skin. The probe measures hemodynamic parameters such as hemoglobin (Hb) concentration, tissue oxygen saturation (StO2), and tissue perfusion. Measures of vascular reactivity and endothelial function in the lower limbs can provide a rapid and direct method for assessing the extent of severity. To date, three patients have been recruited for the study.
NCT05072483 – Natural History Study of CADASIL
CADASIL is a monogenic small-vessel disease that affects the arteries in the brain. Clinical manifestations include migraines, recurrent strokes, and progressive white matter degeneration. Current diagnosis for CADASIL is done through MRI imaging of the white matter, genetic testing, or through family history. Previous literature reported impaired cerebrovascular reactivity detected by MRI and transcranial doppler. NIRS is an attractive alternative method for assessing cerebrovascular reactivity because of its compact size and penetration depth. For the CADASIL cohort, optical probes will be affixed to the forehead and forearm to acquire information from the prefrontal cortex and skeletal muscle respectively. The optical measurement consists of a five-minute baseline period followed by a five-minute brachial cuff occlusion and a five-minute recovery period. A series of three end-exhalation breath holds follow the recovery period; patients will be asked to exhale and hold their breaths for either 30 seconds or for as long as they can tolerate. We anticipate that these assessments of cerebrovascular compared with skeletal muscle reactivity could provide a predictive test of disease progression. To date, five patients have been enrolled in the study.
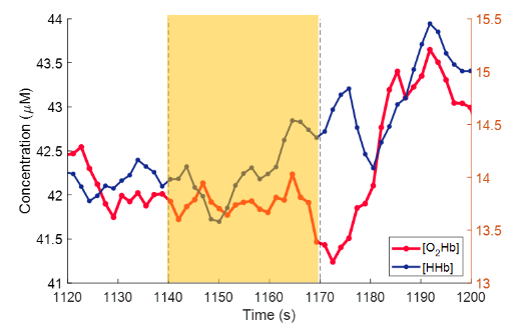
Figure 4. TD-NIRS trace of [O2Hb] and [HHb] concentrations of a CADASIL patient during a breath hold
Trace of the [O2Hb] (red) and [HHb] (blue) concentrations in the forehead and forearm of a CADASIL patient during a breath hold (highlighted in yellow). During each breath hold, there does not appear to be a discernable increase in [O2Hb]. Three breath holds were performed for each patient for a total of nine breath holds across the three patients recruited. The median slope of [O2Hb] during the breath hold period was 0.0029 ± 0.06 µM/s. Previous literature evaluating NIRS as a candidate for assessing cerebrovascular reactivity reports a gradual increase in [O2Hb] during a breath hold. This lack of a cerebrovascular response can be observed in the other two patients in the cohort. Prior literature has suggested that cerebrovascular reactivity could a predictive test of disease progression. To date, three patients have been enrolled in the study.
NCT04595773 - COVID-19 - Chronic Adaptation and Response to Exercise (COVID-CARE): a randomized controlled trial
While COVID-19 is mainly perceived as a respiratory illness, there is also significant evidence of vascular complications, especially in those with severe symptoms and long-term effects. In collaboration with NHLBI's TVMB and the NIH Clinical Center’s Rehabilitation Medicine Department, we have performed optical hemodynamic assessments in a cohort of patients recovering from COVID. The measurements are one component of a larger randomized clinical study evaluating the efficacy of targeted exercise intervention to improve recovery from COVID. Patients recruited for this study are randomized into either a control or treatment arm. Patients in the treatment arm undergo a ten-week exercise regimen after their baseline visit, followed by a follow-up visit. Patients in the control arm continue with their typical daily routine for ten weeks after the baseline visit and return for a follow-up visit; patients then begin the ten-week exercise regimen followed by another follow-up visit. Patients recruited into the study undergo a brachial cuff occlusion and also participate in a set of breath holding exercises. In a separate measurement session, patients undergo an exercise test during which optical measures are acquired before and after exercise. As the study is ongoing, analysis is currently limited to comparing optical measures to established clinical metrics. We anticipate that a more comprehensive analysis will be performed upon conclusion of the study.
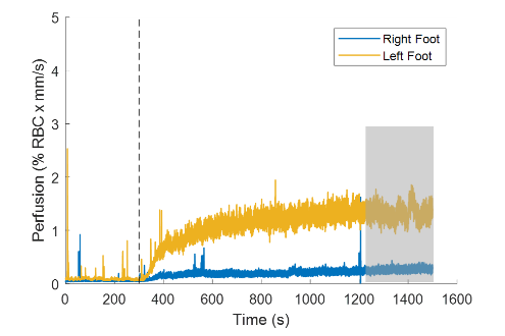
Figure 5. Perfusion on the right (blue) and left (yellow) foot of a patient with ACDC during a thermal hyperemia challenge. Black dotted line denotes when probe temperature was increased to 44°C.
Total perfusion measured on both feet from a patient with ACDC. Following the baseline period, the perfusion response can be observed in two phases. The first phase is a rapid increase within the first few minutes of the temperature rise, followed by a more gradual increase that eventually plateaus towards the end of the measurement. We define a mean perfusion level during the last five minutes of the measurement in the plateau region as the plateau perfusion. We compared the plateau perfusion in the ACDC cohort with previous literature, which compared the plateau perfusion in a patient with diabetes with age-matched healthy volunteers. Plateau perfusion responses in the ACDC cohort (n = 3, 0.91 ± 0.50% RBC x mm/s) were more similar in magnitude to the diabetic cohort (1.09 ± 0.50% RBC x mm/s) than the healthy volunteer cohort (1.60 ± 0.98% RBC x mm/s). To date, three patients have been recruited for the study, and additional patients with ACDC are anticipated.
Continuous blood pressure and vascular monitoring technologies for optimizing maternal health
Pre-eclampsia is a pregnancy-specific disorder responsible for more than 70,000 maternal deaths and 500,000 fetal deaths worldwide every year. While high blood pressure is the main clinical phenotype, pre-eclampsia can trigger several hemodynamic changes, including increased vascular stiffness and reduced vascular reactivity. While delivery can resolve most symptoms, they can persist postpartum and continue to impact the mother. Early diagnosis combined with treatment remains a key component to improving patient outcomes. However, the transition from gestational hypertension to mild pre-eclampsia to severe pre-eclampsia is a dynamic process; early diagnosis would ideally necessitate continuous, heightened surveillance. There is a need for technologies that can easily monitor the hemodynamic state of the mother and aid in the detection and risk stratification of pre-eclampsia.
Optical spectroscopic techniques (i.e., NIRS) can provide a broad, point-of-care hemodynamic evaluation by continuously measuring hemodynamic parameters such as vascular reactivity, blood flow, and pulse transit time. There are a limited number of studies evaluating NIRS technologies in pre-eclampsia; Guerci et al. [Crit Care Med 2014;42:2379] measured significantly impaired baseline cerebral oxygenation in patients with severe pre-eclampsia and demonstrated the utility of NIRS in monitoring cerebral oxygen saturation during the administration of magnesium sulfate (MgSO4). A combination of ECG and photoplethysmography (PPG) has been proposed to measure pulse transit time (PTT), the time needed for an arterial pressure wave to pass between two sites. PTT is inversely related to blood pressure and is a potential approach for continuous and cuff-free blood pressure monitoring.
Affixed transmission speckle analysis (ATSA), introduced by our lab in 2018, is a promising candidate to measure blood flow, vascular stiffness, and pulse transit time in a compact form-factor. To recover blood flow information, ATSA sends coherent light through a peripheral digit to measure intensity fluctuations caused by moving red blood cells. The high acquisition speed of ATSA enables the recovery of a pulsatile waveform originating from blood flow, referred to as the speckle plethysmograph (SPG). While previous work with pulse transit time evaluated the feasibility of PPG–based approaches, the SPG offers better signal quality than the PPG, with SPG estimations of heart rate variability having improved accuracy over PPG estimations.
We are developing a compact, multi-modal optical platform that provides continuous, comprehensive hemodynamic assessments at the bedside. The sensor would both acquire information pertaining to cerebral oxygenation as well as PTT derived from the SPG signal. We are first developing a method for calculating PTT with the SPG signal and comparing its ability to estimate BP to PTT calculated with a PPG signal.
In collaboration with Roberto Romero, we recently conducted a pilot study to compare hemodynamic differences between patients with pregnancy complications and patients without complications. Additionally, we monitored a set of patients with pregnancy complications undergoing treatment for their symptoms. These data could help to distinguish patients with preeclampsia from normal physiological variation in healthy controls. The optimal NIRS technique can then be adopted and applied for disease monitoring in pre-eclampsia. Analysis of this study is now under way.
Publications
- Warren RV, Bar-Yoseph R, Hill B, Reilly D, Chiu A, Radom-Aizik S, Cooper DM, Tromberg BJ. Diffuse optical spectroscopic method for tissue and body composition assessment. J Biomed Opt 2022 27:6.
Collaborators
- Ruth Benca, MD, PhD, Wake Forest School of Medicine, Winston-Salem, NC
- Manfred Boehm, MD, Laboratory of Cardiovascular Regenerative Medicine, NHLBI, Bethesda, MD
- Ashura Buckley, MD, Office of the Clinical Director, NIMH, Bethesda, MD
- Leighton Chan, MD, MPH, Rehabilitation Medicine, Clinical Center, NIH, Bethesda, MD
- Courtney Fitzhugh, MD, Cellular and Molecular Therapeutics Branch, NHLBI, Bethesda, MD
- Ingemar Frederiksson, PhD, Perimed Instruments, Järfälla-Stockholm, Sweden
- Amir Gandjbakhche, PhD, Section on Translational Biophotonics, NICHD, Bethesda, MD
- Ramy Khayat, MD, School of Medicine, University of California, Irvine, CA
- Thomas J. Pohida, MS, Instrumentation Development and Engineering Application Solutions, NIBIB, Bethesda, MD
- Forbes Porter, MD, Office of the Clinical Director, NICHD, Bethesda, MD
- Randall Pursley, Instrumentation Development and Engineering Application Solutions, NIBIB, Bethesda, MD
- Samar Rahhal, MD, Office of the Clinical Director, NICHD, Bethesda, MD
- Roberto Romero, MD, DMedSci, Perinatology Research Branch, NICHD, Detroit, MI
- Adam Schiffenbauer, MD, Clinical Research Branch, NIEHS, Bethesda, MD
- Arun Shet, MD, PhD, Sickle Cell Branch, NHLBI, Bethesda, MD
- Hiroaki Suzuki, PhD, Hamamatsu Photonics K.K., Hamamatsu, Japan
- Swee Lay Thein, MB, FRCP, FRCPath, DSc, Sickle Cell Branch, NHLBI, Bethesda, MD
Contact
For more information, email bruce.tromberg@nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/tromberg.