Cell Fusion Stages of Myogenesis and Osteoclastogenesis: Mechanisms and Physiological Role

- Leonid V. Chernomordik, PhD, Head, Section on Membrane Biology
- Eugenia Leikina, DVM, Senior Research Assistant
- Kamram Melikov, PhD, Staff Scientist
- Elena Zaitseva, PhD, Staff Scientist
- Jarred Whitlock, PhD, Intramural Research Training Award Fellow
- Jared Cunanan, BS, Postbaccalaureate Fellow
- Wendy Zhang, BS, Postbaccalaureate Fellow
Diverse biological processes, in which enveloped viruses infect cells and cells from all kingdoms of life secrete, internalize, traffic and sort integral proteins, sculpt their membranes and bring together parent genomes in sexual reproduction, share a common stage: fusion of two membranes into one. Biological membrane remodeling is tightly controlled by protein machinery, but is also dependent on the lipid composition of the membranes. Whereas each kind of protein has its own individual personality, membrane lipid bilayers have rather general properties, manifested by their resistance to disruption and bending and by their charge. Our long-term goal is to understand how proteins fuse membrane lipid bilayers. We expect that better understanding of important fusion reactions will bring about new ways of controlling them and lead to new strategies for quelling diseases involving cell invasion by enveloped viruses and defects in intracellular trafficking or intercellular fusion. Our general strategy is to combine in-depth analysis of the best characterized fusion reactions with comparative analysis of diverse less explored fusion reactions that can reveal new kinds of fusion proteins and clarify the generality of emerging mechanistic insights. In our recent studies, we explored the mechanisms of myoblast fusion and osteoclast fusion in development and regeneration of muscles and bones.
Myomerger promotes fusion pore formation by elastic coupling between proximal membrane leaflets and hemifusion diaphragm.
Myoblast fusion, a key stage in the development and regeneration of skeletal muscle, is a multistep process, starting from hemifusion controlled by the muscle-specific protein Myomerger/Myomixer/Minion and followed by opening of a fusion pore controlled by another muscle-specific protein, Myomaker [reviewed in Petrany MJ, Millay DP. Trends Cell Biol 2019;29:964-973]. Molecular mechanisms by which Myomerger, a single-pass transmembrane protein containing 84 amino acids with an ectodomain that includes two alpha helices drives progression beyond early hemifusion events to complete fusion, remain to be understood.
In our earlier studies [Leikina E. et al. Dev Cell 2018;46:767-780; Reference 5], we demonstrated that Myomerger acts by destabilizing membranes through generation of elastic stresses in the outer leaflet of the plasma membrane. In more recent work [Reference 3], we continued our collaboration with Douglas Millay’s lab to explore the specific contributions of different domains of Myomerger to functionally important protein-lipid bilayer interactions. We also examined how phosphatidylserine, a lipid previously implicated in diverse cell-cell fusion processes, including myoblast fusion [reviewed in Reference 4], regulates Myomerger activity. Under fusion conditions, phosphatidylserine, normally present only in the inner leaflet of the plasma membrane, is also transiently exposed in the outer membrane leaflet. Mechanisms that couple the phosphatidylserine signaling with the function of the fusion protein machinery are yet unclear. We focused on the membrane interactions of different domains of Myomerger and the dependence of these interactions on the lipid composition of membrane lipid bilayer. Complementing myoblast fusion assays and in vitro liposome assays, we found that the two helices possess unique characteristics, which are needed for full activity of the protein. We characterized membrane insertion of different regions of Myomerger by evaluating changes in the spontaneous curvature of the lipid monolayer. To characterize changes in this membrane property, which is critically important for membrane fusion, we used our recently published assay, in which we compare the effects of inserted molecules on the FRET efficiency between dyes attached to either lipid headgroups or lipid tails [Reference 5]. We found that the membrane-proximal, amphipathic Helix-1 is normally disordered, and that its alpha-helical structure is induced by phosphatidylserine, facilitating interactions of this region with the membrane. The distal, more hydrophobic Helix-2 is intrinsically ordered, possesses an ability to insert into membranes, and promotes the membrane-stressing effects of Helix-1. The removal of either of the two alpha helices in the Myomerger ectodomain dramatically reduced its effect on Forster resonance energy transfer, suggesting that both alpha helices are necessary for efficient insertion and curvature generation. Similarly, mutations in either of the alpha helices reduced insertion and positive curvature generation. Our findings reveal that Myomerger fusogenic activity is a tightly controlled event involving its two ectodomain helices, which are regulated by changes in the lipid composition of the fusing membranes, providing an explanation as to how its membrane-stressing activity is spatially and temporally regulated during the final stage of myoblast fusion in the formation of multinucleated muscle cells. A deeper understanding of the myoblast fusion mechanism could help develop new therapeutic strategies for genetic and acquired muscle diseases. The uncovered mechanism, by which cells control the activity of proteins that merge membranes, can underlie fusion-pore formation in diverse fusion processes and in other cell-biological processes involving protein-induced membrane deformations.
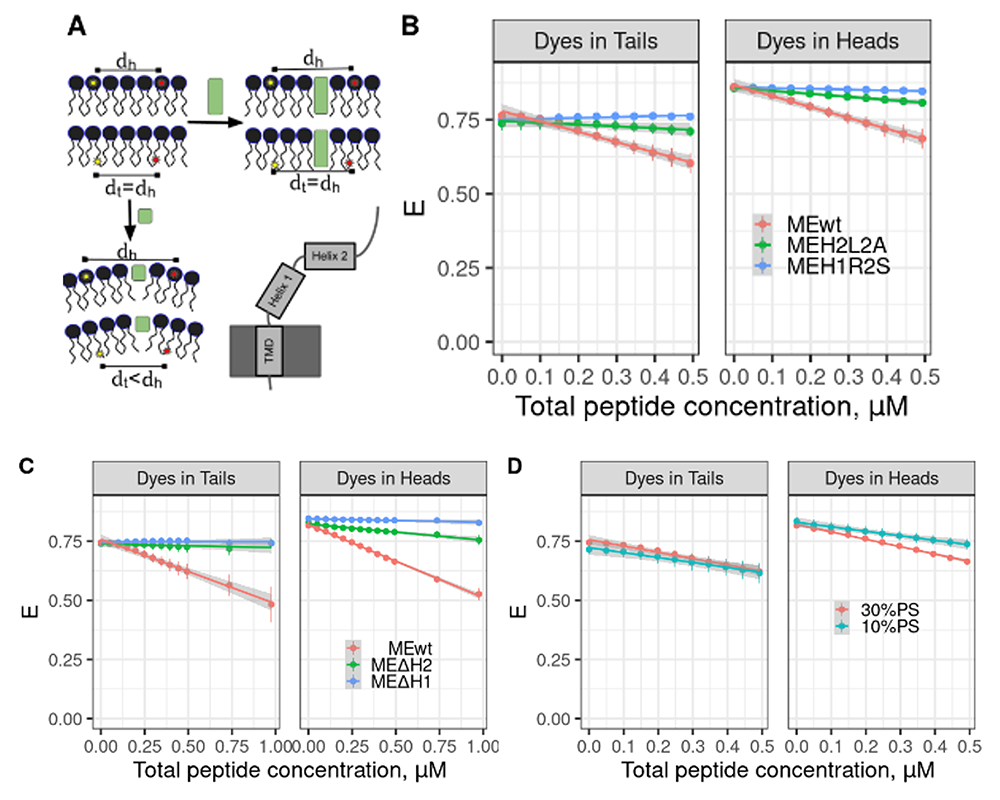
Figure 1. Binding of Myomerger and generation of positive curvature require α-helix domains and depend on phosphatidylserine (PS).
A. In contrast to cylindrical molecules (green rectangles), membrane insertion of molecules generating positive spontaneous curvature (small green square) results in a larger change in the distance between donor and acceptor dye pairs located in the lipid headgroups than for the dye pairs located in the tail region. Domain diagram of Myomerger is shown in the right bottom corner.
B. Mutant Myomerger ectodomain (ME) with R to S substitution in the first α-helix (ME-H1R2S) and mutant with L to A substitution in the second α-helix (ME-H2L2A) induce smaller change in FRET for dyes both in headgroups and in tails compared with wild-type (WT) ME.
C. MEs lacking either the first α-helix (MyoΔH1) or the second α-helix (MyoΔH2) induce smaller change in FRET between dyes both in headgroups and in tails than in WT ME.
D. WT Myomerger induces larger changes in FRET for dyes in lipid headgroups in liposomes containing 30% PS than in those containing 10% PS.
Additional Funding
- Office of AIDS Research Award 2022
- Research on Women's Health (ORWH) through the Bench to Bedside Program award 2022, 2023
Publications
- Li T, Hadigan C, Whitlock JM, Qin J, Kumar J, Kumar P, Catalfamo M. IL-27 modulates the cytokine secretion in the T cell-osteoclast crosstalk during HIV infection. Front Immunol 2022 13:818677.
- Whitlock JM, Leikina E, Melikov K, de Castro LF, Mattijssen S, Maraia RJ, Collins MT, Chernomordik LV. Cell surface-bound la protein regulates the cell fusion stage of osteoclastogenesis. bioRxiv 2022 2022.03.08.479741.
- Gamage DG, Melikov K, Paola Munoz-Tello P, Wherley TJ, Focke LC, Leikina E, Huffman E, Diao J, Kojetin DJ. Prasad V, Chernomordik LV, Millay DP. Phosphatidylserine orchestrates Myomerger membrane insertions to drive myoblast fusion. Proc Natl Acad Sci USA 2022 119:e2202490119.
- Whitlock JM, Chernomordik LV. Flagging fusion: phosphatidylserine signaling in cell-cell fusion. J Biol Chem 2021 296:100411.
- Golani G, Leikina E, Melikov K, Whitlock JM, Gamage DG, Luoma-Overstreet G, Millay DP, Kozlov MM, Chernomordik LV. Myomerger promotes fusion pore by elastic coupling between proximal membrane leaflets and hemifusion diaphragm. Nat Commun 2021 12(1):495.
Collaborators
- Alison Boyce, MD, Metabolic Bone Disorders Unit, NIDCR, Bethesda, MD
- Michael Collins, MD, Skeletal Disorders & Mineral Homeostasis Section, NIDCR, Bethesda, MD
- Michael M. Kozlov, PhD, DHabil, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- Richard Maraia, MD, Section on Molecular and Cellular Biology, NICHD, Bethesda, MD
- Leonid Margolis, PhD, Section on Intercellular Interactions, NICHD, Bethesda, MD
- Douglas Millay, PhD, Cincinnati Children's Hospital Medical Center, Cincinnati, OH
Contact
For more information, email chernoml@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/chernomordik.