Organ and Tissue Formation during Development

- Brant M. Weinstein, PhD, Head, Section on Vertebrate Organogenesis
- Van Pham, BS, Scientific Technician
- Leah Greenspan, PhD, Postdoctoral Fellow
- Aurora Kraus, PhD, Postdoctoral Fellow
- Miranda Marvel, PhD, Postdoctoral Fellow
- Jong Park, PhD, Postdoctoral Fellow
- Laura Pillay, PhD, Postdoctoral Fellow
- Kiyohito Taimatsu, PhD, Postdoctoral Fellow
- Marina Venero Galanternik, PhD, Postdoctoral Fellow
- Keith Ameyaw, BS, Postbaccalaureate Fellow
- John Prevedel, BS, Postbaccalaureate Fellow
- Yehyun Kim, BS, Postbaccalaureate Fellow
- Celia Martinez-Aceves, BS, Postbaccalaureate Fellow
The major focus of the Section is to understand how the elaborate networks of blood and lymphatic vessels arise during vertebrate development. Blood vessels supply every tissue and organ with oxygen, nutrients, and cellular and humoral factors. Lymphatic vessels drain fluids and macromolecules from the interstitial spaces of tissues, returning them to the blood circulation, and they play an important role in immune responses. Our studies on the formation of blood and lymphatic vessels are of great clinical interest because of the roles that both types of vessel play in pathologies such as cancer and ischemia.
The zebrafish (Danio rerio), a small tropical freshwater fish, possesses a unique combination of features that make it particularly suitable for studying vessel formation. Zebrafish are genetically tractable vertebrates with externally developing, optically clear embryos, which are readily accessible to observation and experimental manipulation, features that permit observation of every vessel in the living animal and simple, rapid screening for even subtle vascular-specific defects (Figure 1). Our current studies use genetic screening, experimental analysis, and imaging to examine cues directing vascular patterning and morphogenesis, regulation of vascular integrity, assembly of the lymphatic system, and the roles of novel vascular-associated cells.
As a second major effort in addition to our work on vessel development, we are pursuing studies on the role of epigenetics during early development, in particular how DNA methylation and other epigenetic mechanisms help coordinate cell, tissue, and organ specification and differentiation, using a novel ‘EpiTag’ epigenetic reporter line and the first large-scale genetic screen for tissue-specific epigenetic regulators in a vertebrate organism.
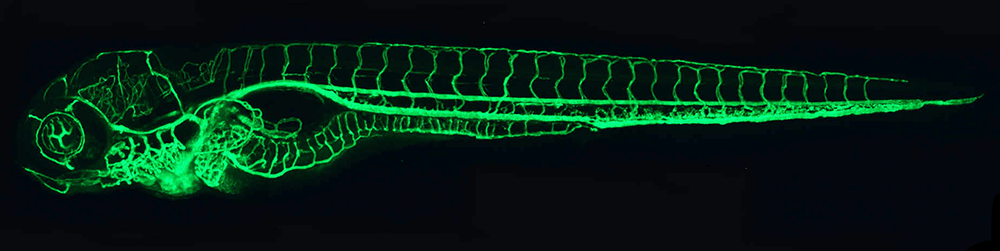
Figure 1. The zebrafish vascular system
Confocal micro-angiogram of the vascular system of a 4½-day-old zebrafish larva labeled by injecting fluorescent microspheres. The transparency of zebrafish larvae makes it possible to use high-resolution optical imaging methods to visualize the entire vasculature in exquisite detail.
Specification and patterning of developing blood vessels
We are working to elucidate the cellular and molecular mechanisms responsible for the specification, patterning, and differentiation of blood vessels during development. Blood vessels are ubiquitous and vital components of vertebrate animals, innervating and supplying every tissue and organ with oxygen and nutrients. Many of the recent insights into mechanisms of blood vessel formation have come from studies in model organisms, including the zebrafish. Using the fish, we are carrying out several related projects, which are described below.
New tools for experimental analysis of vascular development
We generate novel transgenic lines for visualizing different types of endothelial and perivascular cells, and for driving gene expression or performing molecular profiling of mRNAs and microRNAs in these cell populations.
Genetic analysis of vascular development
We have identified many novel mutants affecting vascular development in our transgene-assisted forward-genetic screens and are currently characterizing the phenotypes and molecular basis for several of the mutants.
Analysis of vascular specification, patterning, morphogenesis, and function
We are studying the development and function of several vascular beds, including the vasculature of the gills, the fish equivalent of the mammalian lungs, which contains unique endothelial cell populations and plays important roles in gas exchange.
Regulation of vascular integrity
We are using the zebrafish to understand the cellular and molecular mechanisms responsible for proper vessel morphogenesis and for the generation and maintenance of vascular integrity. Disruption of vascular integrity is associated with hemorrhagic stroke, a severe and debilitating form of stroke associated with high morbidity and mortality. Meningeal vascular dysfunction is also associated with neuro-cognitive deficits and neuro-degenerative disease. Many of the recent insights into the molecular mechanisms regulating vascular integrity have come from studies in model organisms such as the zebrafish. We are pursuing several related projects.
Genes regulating vascular integrity
With forward-genetic screens we identify new zebrafish mutants that disrupt cranial vascular integrity in the zebrafish (Figure 2), using next-gen sequencing methods to accomplish higher throughput cloning of mutants. We already characterized the role of GDF6 (growth differentiation factor 6, also known as BMP13) in vascular integrity, demonstrating that the gene promotes maintenance of vascular integrity by suppressing excess VEGF (vascular endothelial growth factor) signaling. We recently characterized the role of RHOA in vascular integrity and angiogenesis. This small GTPase–regulatory protein has been shown to be involved in cytoskeletal dynamics, transcription, cell-cycle progression, and cell transformation, and a precisely calibrated level of RHOA signaling is required for proper vascular growth and function, with either too little or too much RHOA signaling resulting in vascular-integrity and angiogenesis defects.
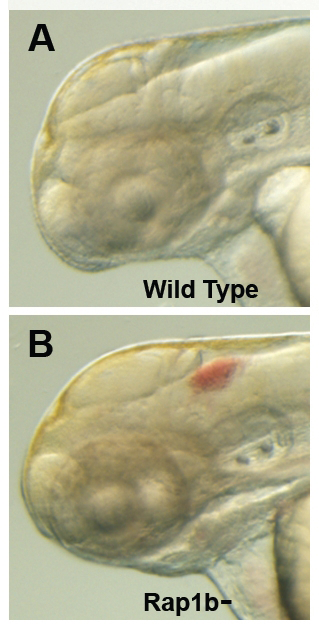
Figure 2. Intracranial hemorrhage (ICH) in the developing zebrafish
The clarity of zebrafish larvae also makes it straightforward to screen for animals with intracranial hemorrhage, as is evident in comparing lateral views of a 2-day-old wild-type larva (A) with a hemorrhage-prone larva deficient in rap1b (B).
Revascularization after injury
Vascular regrowth and remodeling are critical for proper wound healing, and defective vessel growth in cutaneous wounds is associated with delayed wound repair and/or chronic open wounds susceptible to infection. We developed a new zebrafish model of vascular regrowth and repair after cutaneous injury in adult zebrafish, and we are using this model to understand the cellular and molecular changes in that lead to defective vascular responses to injury and poor wound healing in aging or diabetes, with an eye toward uncovering new therapeutic targets to promote improved revascularization and healing in these contexts.
Vasculature and vascular-associated cells in the meninges
The meninges are an external, enveloping connective tissue that encases the brain, producing cerebrospinal fluid, acting as a cushion against trauma, nourishing the brain via nutrient circulation, and removing waste. Despite its importance, the cell types present in the meninges and its function and embryonic origins are still not well understood. We recently discovered and characterized fluorescent granular perithelial cells (FGPs) in the zebrafish, a novel endothelium-derived perivascular cell population closely associated with meningeal blood vessels, which is likely to play a critical role in meningeal function (Figure 3). As discussed further below, we also discovered a bona fide meningeal lymphatic vascular network in the zebrafish. We are currently carrying out additional comprehensive anatomical and molecular studies to understand the structure and cellular composition of the zebrafish meninges and the function of FGPs, meningeal lymphatics, and other novel meningeal cell populations.
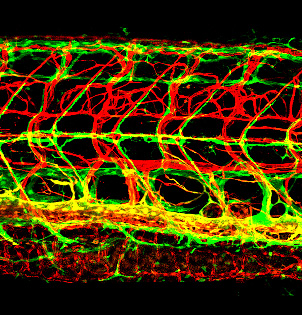
Figure 3. Novel perivascular cells on the zebrafish brain
Confocal micrograph of fluorescent granular perithelial cells (FGPs, green) adhering to the outside of meningeal blood vessels (red) on the brain of a Tg(mrc1a:egfp);Tg(kdrl:cherry) double-transgenic adult zebrafish. We recently showed that FGPs are unique endothelium-derived perivascular cells with unusual scavenging properties that are likely to be critical for brain homeostasis.
Specification and patterning of the lymphatic system
The lymphatic system is a vascular system completely separate from the blood circulatory system consisting of an elaborate blind-ended tree of vessels that extends through most of the body, emptying lymph fluid into the venous blood vascular system via several evolutionarily conserved drainage points. The lymphatic system is essential for immune responses, fluid homeostasis, and fat absorption, and is involved in many pathological processes, including tumor metastasis and lymphedema. However, progress in understanding the origins and early development of the system has been hampered by difficulties in observing lymphatic cells in vivo and performing defined genetic and experimental manipulation of the lymphatic system in currently available model organisms. Our ground-breaking studies demonstrated that zebrafish possess a lymphatic system that shares many of the morphological, molecular, and functional characteristics of lymphatic vessels found in other vertebrates, providing a powerful model for the purposes of imaging and studying lymphatic development. We are currently pursuing further study of the formation of the lymphatic system through several ongoing projects.
- We generated new transgenic lines that permit direct, specific visualization, and tissue-specific molecular profiling of developing lymphatic vessels and are using these transgenic animals to further characterize lymphatic development (Figure 4).
- We carried out forward-genetic ENU (N-ethyl-N-nitrosourea) mutagenesis screens, using our lymphatic reporter transgenic lines to identify lymphatic-specific mutants with defects in novel genes that play important roles in lymphatic development.
- We characterized and studied novel microRNAs expressed in the lymphatic endothelium and how these small regulatory RNAs influence lymphatic gene expression and lymphatic development.
- We discovered a previously unreported lymphatic network in the dural meninges of the zebrafish (Figure 5). Like similar recently discovered meningeal lymphatics surrounding the mammalian brain, the zebrafish network is likely to play critical role in maintaining homeostasis and protecting the brain from mechanical trauma and infection, and we are carrying out a detailed analysis of the development, form, and function of these critical vessels and their role in immune cell interaction and trafficking.
Figure 4. Novel lymphatic vascular reporter
Lateral view confocal image of the trunk of a 12 dpf (days post-fertilization) Tg(kdrl:cherry); Tg(mrc1a:egfp) double-transgenic zebrafish with red fluorescent blood vessels and green fluorescent lymphatics. See Jung HM, et al. Development 2017;144:2070 for additional details.
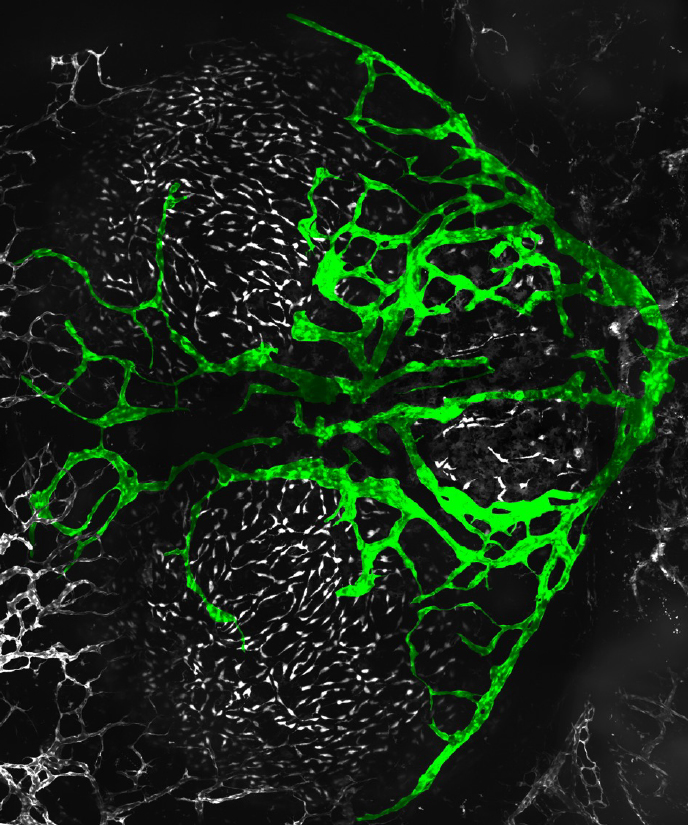
Figure 5. Adult zebrafish meningeal lymphatic network
Confocal image of the dorsal head of a Tg(mrc1a:egfp)y251, casper adult zebrafish, with mrc1a+FGPs and superficial lymphatics in grey and intracranial meningeal lymphatics pseudocolored green. Anterior is to the left, right is up.
The results of our studies, combining the genetic and experimental tools available in the zebrafish with the ability to perform high-resolution microscopic imaging of developing vascular structures in living animals, will continue to lead to important new insights into the origins and growth of the lymphatic system and molecular mechanisms that are critical in lymphatic development and lymphatic pathologies.
Epigenetics of development
We are using the genetically and experimentally accessible zebrafish and Mexican tetra (Astyanax mexicanus, also known as the blind cave fish) models to uncover the molecular basis for organ- and tissue-specific epigenetic regulation during development in the following interrelated projects:
Forward-genetic screen for epigenetic regulatory factors
Genetic screens carried out in Drosophila and the nematode Caenorhabditis elegans have been highly successful in identifying genes regulating cell type–specific epigenetic gene regulation in invertebrates, but the molecular mechanisms involved in organ- and tissue-specific epigenetic regulation in vertebrates are still relatively unknown. We developed a novel zebrafish transgenic reporter line that allows us to monitor dynamic changes in epigenetic regulation in intact animals during development. Using the transgenic line, we performed the first large-scale F3 genetic screen in a vertebrate to identify recessive mutants in regulators of epigenetic gene silencing or activation (Figure 6). Among other mutants, the screen yielded epigenetic regulators of liver development, pharynx development, and arterial differentiation, and we are currently pursuing follow-up studies on these mutants.
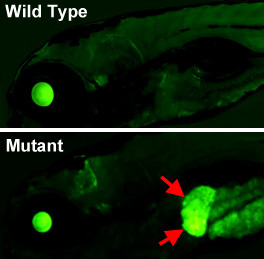
Figure 6. An epigenetic silencing mutant in the zebrafish
Lateral views of the head and anterior trunk of a wild-type (top) and tissue-specific epigenetic silencing mutant (bottom) zebrafish. The mutation causes loss of epigenetic silencing specifically in the liver (red arrows), as visualized with a novel transgenic reporter line developed in our lab, which permits dynamic, tissue-specific visualization of epigenetic silencing in living animals.
Molecular mediators of glycemic memory in diabetic vasculopathy
The global burden of diabetes has risen dramatically, with projections that more than 600 million adults will be affected by 2030. Micro- and macrovascular complications in patients with diabetes are the major causes of cardiovascular mortality, renal failure, blindness, and non-traumatic amputations. Diabetes-related complications can emerge even many years after the blood sugar level levels have been brought under control, a phenomenon known as ‘glycemic memory.’ Although the cause of the phenomenon remains to be elucidated, epigenetic alterations in endothelial cells (ECs) may be responsible for the perdurance of diabetic vascular effects. We are using the zebrafish as an in vivo model to examine whether short-term exposure to hyperglycemia results in persistent transcriptomic and epigenomic changes in endothelial cells, even after return to normo-glycemic conditions. We identified several genes with significantly altered endothelial transcription and methylation levels during hyperglycemia that persist during the memory phase. We are currently carrying out further investigation of these ‘glycemic memory loci’ by a variety of methods. Unveiling the epigenetic and transcriptomic landscape of glycemic memory in ECs may lead to better identification of molecular targets and, potentially, to the design of personalized, epigenetic-based therapies to alleviate the enormous burden of diabetic vasculopathy.
Epigenetic regulation of fat and muscle development in cavefish
In addition to eye and pigment loss and other adaptations, Astyanax cavefish (Figure 7) have extreme and unusual metabolic adaptations that allow them to survive chronic and long-term food deprivation, including excess fat deposition, altered liver function, and resistance to metabolic disease. We hypothesize that, in a similar manner to loss of eyes, changes in epigenetic gene regulation may also underlie cavefish metabolic adaptations. We are using single-cell profiling to investigate differences in adipocytes and other cell types in the muscles (where in cavefish there are large amounts of stored fat) and livers of cavefish and surface fish. We are also performing whole-genome bisulfite sequencing and RNA-Seq from surface and cavefish muscles and livers to identify differentially expressed and methylated genes. We will follow up on these findings to elucidate how differential DNA methylation influences fat metabolism and obesity.
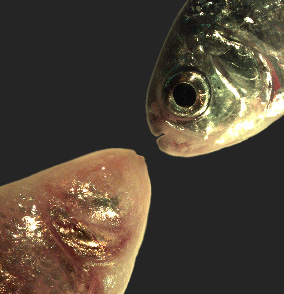
Figure 7. Mexican tetra cave- and surface fish
The Mexican tetra Astyanax mexicanus is a freshwater fish native to parts of southern Texas and eastern and central Mexico, which exists in both surface-dwelling (‘surface morphs,’ top right) and very closely related cave-dwelling (‘cave morphs,’ bottom left) populations. Cave morphs have a series of uniquely evolved adaptations, including loss of eyes and pigment, dramatically altered metabolism, altered vascular function, and altered sleep regulation and behavior. Results from our laboratory suggest that altered DNA methylation and resulting coordinated changes in expression of large sets of genes have helped drive at least some of this rapid evolutionary change.
Additional Funding
- K99/R00 Award (to M. Venero Galanternik)
- Japan Society for the Promotion of Science (JSPS) Award (to K. Taimatsu)
- NICHD Intramural Research Fellowship (to Miranda Marvel)
- NICHD Intramural Research Fellowship (to Jong Park)
- NICHD Early Career Award (to Miranda Marvel)
- NICHD Early Career Award (to Leah Greenspan)
- NICHD Early Career Award (to Kiyohito Taimatsu)
Publications
- Castranova D, Samasa B, Jung HM, Venero Galanternik M, Weinstein BM. Live imaging of intracranial lymphatics in the Zebrafish. Circ Res 2020 128:42–58.
- Ma L, Ng M, Shi J, Gore AV, Castranova D, Weinstein BM, Jeffery WR. Maternal control of visceral asymmetry evolution in Astyanax cavefish. Sci Rep 2021 11:10312.
- Pillay LM, Yano JJ, Davis AE, Butler MG, Ezeude MO, Park JS, Barnes KA, Reyes VL, Castranova D, Gore AV, Swift MR, Iben JR, Kenton MI, Stratman AN, Weinstein BM. In vivo dissection of Rhoa function in vascular development using zebrafish. Angiogenesis 2022 25:411–434.
- Paulissen SM, Castranova DM, Krispin SM, Burns MC, Menéndez J, Torres-Vázquez J, Weinstein BM. Anatomy and development of the pectoral fin vascular network in the zebrafish. Development 2022 149:dev199676.
- Castranova D, Samasa B, Venero Galanternik M, Gore AV, Goldstein AE, Park JS, Weinstein BM. Long-term imaging of living adult zebrafish. Development 2022 149:dev199667.
Collaborators
- Andreas Baxevanis, PhD, Computational and Statistical Genomics Branch, NHGRI, Bethesda, MD
- Harold Burgess, PhD, Section on Behavioral Neurogenetics, NICHD, Bethesda, MD
- George Davis, PhD, University of Missouri-Columbia, Columbia, MO
- Elisabetta Dejana, PhD, The FIRC Institute of Molecular Oncology Foundation, Milan, Italy
- James Iben, PhD, Molecular Genomics Laboratory, NICHD, Bethesda, MD
- Sumio Isogai, PhD, Iwate Medical University, Morioka, Japan
- William R. Jeffery, PhD, University of Maryland, College Park, MD
- Paul Liu, MD, PhD, Genetics and Molecular Biology Branch, NHGRI, Bethesda, MD
- Richard Maraia, MD, Section on Molecular and Cell Biology, NICHD, Bethesda, MD
- Yoh-suke Mukouyama, PhD, Laboratory of Stem Cell and Neuro-Vascular Biology, NHLBI, Bethesda, MD
- Lisa M. Price, PhD, Division of Developmental Biology, NICHD, Bethesda, MD
- Radu V. Stan, MD, PhD, Geisel School of Medicine at Dartmouth, Lebanon, NH
Contact
For more information, email weinsteb@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/weinstein.