Neuregulin-ErbB and NMDA Receptor Signaling in Neuronal Development and Psychiatric Disorders

- Andres Buonanno, PhD, Head, Section on Molecular Neurobiology
- Detlef Vullhorst, PhD, Staff Scientist
- Eastman Lewis, PhD, Postdoctoral Intramural Research Training Award Fellow
- Mara Bloom, BS, Postbaccalaureate Fellow
- Hayli Spence, BS, Postbaccalaureate Fellow
- Jacqueline Welday, BS, Postbaccalaureate Fellow
- Ricardo Murphy, BS, Contractor
Failure of cortical microcircuits to properly regulate excitatory-inhibitory (E-I) balance is a key feature in the etiology of several developmental psychiatric disorders and neurological diseases, such as schizophrenia, autism, ADHD and epilepsy. E-I balance is important to synchronize the firing pattern of local neuron ensembles, and its dysregulation can degrade cognitive functions and, in extreme cases, result in epileptiform activity. Alterations in neuronal network activity, in particular oscillations in the gamma-frequency range (30–80 Hz), are associated with behavioral and cognitive deficits in psychiatric disorders. We have been investigating whether and how Neuregulins (NRG 1-3) and their major neuronal receptor ErbB4, which are genetically linked to psychiatric disorders, function in an activity-dependent fashion (i.e., experience) in the developing brain to regulate synaptic and neuronal network properties. We use genetically modified NRG and ErbB4 mouse models, in combination with optogenetic, electrophysiological, behavioral, and molecular/cellular techniques, to identify novel interactions between the NRG/ErbB4, glutamatergic, dopaminergic, and GABAergic signaling pathways associated with psychiatric disorders.
Our earlier studies demonstrated that NRG/ErbB4 signaling in GABAergic fast-spiking parvalbumin-positive (PV+) interneurons regulates E-I balance, gamma oscillation network activity, and numerous behaviors relevant to psychiatric disorders. To understand how NRGs mediate their biological functions during brain development, we investigated how different NRG ligands are proteolytically processed and trafficked in neurons, using molecular, cellular, and genetic approaches. NRGs are synthesized as unprocessed pro-proteins (proNRGs) containing either a single or two (dual) transmembrane (TM) domains. Contrary to dogma, we discovered that these two types of NRGs are processed and trafficked very differently. Single–TM NRGs are cleaved and shed from endoplasmic reticulum–plasma membrane (ER–PM) junctions on neuronal soma in an activity-dependent fashion in response to calcium entry through NMDA receptors (NMDAR), a ligand-gated channel that binds the excitatory neurotransmitter glutamate (Figure 1). By contrast, dual–TM NRGs are constitutively cleaved by the protease BACE1 in the trans-Golgi network, sorted into axons by transcytosis, and then selectively retained at presynaptic glutamatergic terminals via juxtacrine trans-synaptic interactions with ErbB4 receptors on dendrites of GABAergic interneurons (Figure 1). The findings suggest that single–TM NRGs signal in paracrine mode at neuronal soma and proximal dendrites, whereas dual-pass TM NRGs signal in juxtacrine fashion from axons and presynaptic terminals by interacting with postsynaptic ErbB4 on GABAergic dendrites.
We investigated how NRG/ErbB4 signaling affects networks and behaviors associated with psychiatric disorders. The prefrontal cortex (PFC) is a site of convergence of long-range glutamatergic inputs that integrate multiple modalities of information to produce goal-directed behaviors. GABAergic PV+ interneurons coordinate pyramidal cell firing induced by this converging glutamatergic innervation to synchronize cortical networks that modulate goal-oriented behaviors that are disrupted in psychiatric disorders. Interestingly, drugs (i.e., ketamine) that inhibit NMDAR activity alter E-I balance, increase gamma oscillation network activity and disrupt behaviors associated with psychiatric disorders. Several lines of evidence suggest that NMDAR antagonists may disproportionally act on NMDARs expressed on PV+ interneurons; however, there has been significant controversy as to whether adult PV+ interneurons have functional NMDARs. Given the importance of PV+ interneurons for cortical functions, and our prior findings that NRG/ErbB4 signaling inhibits NMDAR activity in GABAergic interneurons, we embarked on an investigation into the proportion of PFC adult PV+ interneurons expressing functional NMDARs and their role in local network activity.
Figure 1. Distinct subcellular distribution of NRG2 and NRG3 in a cultured hippocampal ErbB4+ GABAergic interneuron
A dissociated hippocampal GABAergic interneuron showing pro-NRG2 surface puncta (white) clustered at ER-PM contacts and processed NRG3 at presynaptic terminals (green) that are juxtaposed to postsynaptic ErbB4 receptors (red). Note the restricted distribution of pro-NRG2 on the soma and proximal dendrites that correspond to clusters of NRG2 at ER–PM junctions, as confirmed by electron microscopy (inset). In stark contrast to NRG2, NRG3 and ErbB4 staining overlap extensively, consistent with their trans-synaptic interactions at excitatory synapses onto inhibitory interneurons (see also Figure 2).
Presynaptic accumulation of NRG3 in central neurons is achieved by trans-synaptic retention, a novel mechanism for polarized axonal expression.
How stable axonal polarity is maintained remains a central question in neuroscience. We recently demonstrated that dual–TM proNRGs, comprising CRD-NRG1 type III and NRG3, are targeted to axons and accumulate at glutamatergic presynaptic terminals, where they signal in juxtacrine mode via postsynaptic ErbB4 receptors expressed at postsynaptic densities on GABAergic interneurons (Vullhorst, Ahmad et al., J Neurosci 2017;37: 5232). In a new series of studies, we aimed to understand how and where proNRG3 is cleaved, and how its biologically active NRG3 peptide is sorted to and then retained in axons. To this end, we investigated the spatial-temporal dynamics of NRG3 processing and sorting in neurons using an optogenetic proNRG3 cleavage reporter (LA143-NRG3) that we had developed. In dark conditions, unprocessed LA143-NRG3 is retained in the trans-Golgi network (TGN) but, following blue-light photoactivation, it is cleaved by BACE1 and released from the TGN. We found that mature NRG3 initially emerges on the somatodendritic plasma membrane, from where it is re-endocytosed and anterogradely transported on GTPase Rab4–positive vesicles into axons via a process known as transcytosis. Interestingly, our work showed that NRG3 accumulation at axonal presynaptic terminals is mediated by interactions with ErbB4 receptors expressed by postsynaptic GABAergic interneurons. We then went on to demonstrate that the continuous interaction between the NRG3 EGF–like domain and its receptor ErbB4 is necessary for NRG3 retention at presynaptic sites, as either addition of a competing peptide or knock-down of ErbB4 from GABAergic neurons prevents its accumulation (Figure 2). We call this mechanism “trans-synaptic retention” and propose that it accounts for polarized expression of other neuronal transmembrane ligands and receptors [Reference 1].
Figure 2. Diagram summarizing the results of our NRG3 trafficking studies
The diagram depicts the different steps of pro-NRG3 processing that begin with its synthesis as a dual–TM protein in the endoplasmic reticulum, its cleavage, and sorting of the biologically active single–TM NRG3 ligand to axonal presynaptic terminals, where it is retained by virtue of its interaction with postsynaptic ErbB4 receptors. The three major steps of pro-NRG3 processing and axonal retention are:
- Unprocessed dual–TM pro-NRG3 requires cleave by the protease BACE1 to release the biologically active single–TM NRG3 from the trans-Golgi network (TGN).
- NRG3 then traffics to axons by transcytosis, a process that involves initial transport of NRG3 to the somatodendritic plasma membrane, re-endocytosis into Rab5–positive early endosomes, sorting and anterograde axonal transport in Rab4–positive vesicles.
- NRG3 is retained selectively at axonal terminals by virtue of its binding across the synapse to its cognate receptor ErbB4, which is expressed at glutamatergic postsynaptic densities on dendrites of GABAergic interneurons. We termed this mechanism, which is necessary for the polarized expression of NRG3 in axonal terminals, “trans-synaptic retention,” and we propose that it accounts for the polarized expression of other neuronal transmembrane ligands and receptors in axons.
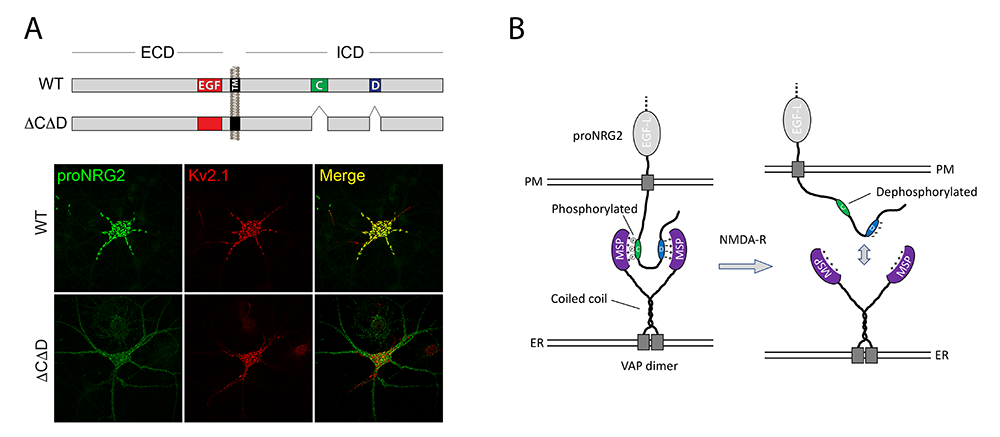
ER–PM junctions on GABAergic interneurons are organized by neuregulin 2/VAP interactions and regulated by NMDA receptors.
Two major unresolved questions we recently pursued were to understand at a mechanistic level: (1) how proNRG2 clusters at ER-PM junctions; and (2) how proNRG2 dissociates from the junctions in response to NMDAR activation. We found that proNRG2 promotes the formation of ER–PM junctions in hippocampal GABAergic interneurons via interactions of its cytoplasmic tail with the ER–resident protein VAP. Interestingly, there are two stretches of amino acids in the intracellular cytoplasmic domain conserved between proNRG1 and proNRG2, denoted C- and D-boxes, that are required to stabilize proNRG2/VAP complexes during immunoprecipitation. Although the protein sequence of neither box conforms to known FFAT motifs, shown in other proteins to bind to VAP, the proNRG2 D-box contains a track of acidic residues required for VAP binding and the C-box harbors a cryptic, phosphorylation-dependent VAP binding site. Importantly, NMDAR activation stimulates dephosphorylation of Ser/Thr residues in the C-box and its dissociation from VAP, which reduces proNRG2 clustering at ER–PM junctions [Vullhorst et al., under review]. These observations are interesting because, although both proNRG2 and the potassium channel Kv2.1 are colocalized at ER–PM junctions and clustering at these sites is regulated by NMDA receptor activity, their modes of interaction with VAP differ (Figure 3). Based on these findings, we hypothesize that autocrine NRG2/ErbB4 signaling and Kv2.1 function synergistically as a homeostatic protective mechanism to downregulate GABAergic interneuron excitability during periods of strong excitatory activity and/or elevated extracellular glutamate levels, which would help to protect these neurons from excitotoxicity.
Figure 3. ProNRG2 clustering at ER-PM junctions is mediated by VAP binding and regulated by phosphorylation
A. Cultured hippocampal neurons transduced with AAVs expressing wild-type proNRG2 (top) or proNRG2DCDD lacking the C- and D-boxes (bottom). WT NRG2 (green) forms large clusters at ER–PM junctions (revealed by Kv2.1 staining in red), which are located on the cell body and proximal dendrites. Removal of the VAP binding sites in proNRG2DCDD abolishes its accumulation at ER–PM junctions, causing it to broadly distribute throughout the neuronal plasma membrane.
B. Working model of proNRG2/VAP interactions. ProNRG2 cooperatively engages with a VAP dimer via its two low-affinity FFAT motifs in the C- and D-boxes. Interactions between the VAP major sperm protein (MSP) domain and the cryptic FFAT site in the C-box require Ser/Thr phosphorylation. Their dephosphorylation downstream of NMDAR activation promotes proNRG2 dissociation from VAP.
A bidirectional mechanism that regulates NRG2 processing and NMDA receptor activity in GABAergic interneurons
Single-pass TM NRG1 type II and NRG2 accumulate as unprocessed proforms at ER–PM junctions on neuronal soma and proximal dendrites. We recently demonstrated that calcium entry through NMDA receptors promotes dephosphorylation of serine/threonine residues in the proNRG2 intracellular region, which results in the dissociation of proNRG2 from ER–PM junctions, metalloproteinase ADAM10 cleavage, and the rapid release of the biologically active NRG2 ectodomain, which activates ErbB4 signaling [Vullhorst & Buonanno, Mol Neurobiol 2019;56:8345]. In turn, activation of ErbB4 receptors at excitatory postsynaptic densities of GABAergic interneurons selectively down-regulates NMDA receptor activity [Vullhorst et al., Nat Comm 2015;6:7222]. We hypothesize that this bidirectional NMDAR–NRG2 (up)/ErbB4–NMDAR (down) signaling mode serves as a homeostatic mechanism that regulates the activity of GABAergic interneurons. Importantly, disruption of such a homeostatic mechanism would alter E-I balance and neuronal network activity, consequently affecting numerous psychiatric-relevant behaviors known to be altered in NRG2 and ErbB4 knockout mice [Yan et al. Mol Psychiatry 2018;23:1233; Skirzewski et al., Mol Psychiatry 2018;23:2227; Reference 2].
NRG2 and ErbB4 knockout mice exhibit a dopamine imbalance and behavioral alterations relevant to psychiatric disorders.
NRG2 expression is more widespread than originally reported, extending to striatal and medial prefrontal cortical (mPFC) neurons. Unexpectedly, we found that, in contrast to GABAergic interneurons that express ErbB4 receptors on their soma and dendrites, mesencephalic dopamine (DA) neurons also express ErbB4 on their axons. To investigate the function of NRG2–ErbB4 signaling, we generated NRG2 and ErbB4 knockout (KO) mice. We found that NRG2 and ErbB4 KOs have higher extracellular DA levels in the dorsal striatum but lower levels in the mPFC and hippocampus, a pattern of DA imbalance that recapitulates the reported prefrontal cortical reduction and striatal increase of DA levels in schizophrenia patients. NRG2 and ErbB4 KO mice performed abnormally in a battery of behavioral tasks relevant to psychiatric disorders (Figure 4). They exhibit hyperactivity in a novelty-induced open field, deficits in pre-pulse inhibition, hypersensitivity to amphetamine, antisocial behaviors, reduced anxiety-like behavior in the elevated plus maze, and deficits in the T-maze alteration reward test, a task dependent on hippocampal and mPFC function. In addition, ErbB4 KO mice exhibit reduced spatial learning and memory on the Barnes maze and perform markedly worse in conditioned place preference (CPP) tasks when associating cued-reward palatable food with location. However, we found that the poor performance of ErbB4 KOs in CPP likely results from deficits in spatial memory, instead of reward seeking, as ErbB4 KOs are more motivated to work for palatable food rewards [Reference 2]. Taken together, our work emphasizes the importance of NRG2–ErbB4 signaling in nigrostriatal, mesocortical, and mesolimbic DA systems, and it demonstrates that this signaling pathway regulates a wide array of behaviors relevant to psychiatric disorders, including schizophrenia.
Figure 4. Overlapping behavioral and neurochemical phenotypes in NRG2 and ErbB4 KO mice
Lack of either NRG2 or ErbB4 in genetically engineered mice elicits similar phenotypic alterations with relevance to psychiatric disorders, demonstrating that NRG2 is an important and non-redundant ErbB4 receptor ligand in the postnatal brain.
| NRG2-/- | ErbB4-/- | |
|---|---|---|
| Open Field (Hyperactivity) | increased | increased |
| Elevated Plus Maze (Anxiety) | reduced | reduced |
| Prepulse Inhibition (Sensorimotor Gating) | reduced | reduced |
| T-Maze (Working Memory) | reduced | reduced |
| Amphetamine Sensitivity | increased | increased |
Developmental, neurochemical, and behavioral analyses of ErbB4 Cyt-1 knockout mice
ErbB4 transcripts are alternatively spliced to generate isoforms that either include (Cyt-1) or exclude (Cyt-2) exon 26, an exon that encodes a cytoplasmic domain that imparts to ErbB4 receptors the ability to signal via the PI3K/Akt pathway rather than the MAPK pathway. To investigate the effects of germline (constitutive) and conditional (acute) deletions of the Cyt-1 exon, we generated and studied ErbB4–floxed (ErbB4-Cyt1fl/fl ) mice, because ErbB4 Cyt-1/2 isoforms had only been studied in cultured cells, and clinical genetic studies specifically implicated ErbB4 Cyt-1 variants as a risk factor for schizophrenia. We found that, overall, ErbB4 mRNA levels remain unchanged in germline ErbB4 Cyt-1 knockouts (Cyt-1 KOs), with all transcripts encoding Cyt-2 variants. In contrast to mice lacking all ErbB4 receptor function, GABAergic interneuron migration and number are unaltered in Cyt-1 KOs. However, basal extracellular dopamine (DA) levels in the medial prefrontal cortex are elevated in Cyt-1 heterozygotes. Despite these neurochemical changes, Cyt-1 heterozygous and homozygous mice do not manifest the behavioral abnormalities previously reported to be altered in ErbB4 null mice. To address the possibility that Cyt-2 variants compensate for the lack of Cyt-1 during development, we microinjected an adeno-associated virus expressing Cre-recombinase (AAV-Cre) into the DA–rich ventral tegmental area of adult ErbB4-Cyt1fl/fl mice to acutely target exon 26. Such conditional Cyt-1 KOs were found to exhibit behavioral abnormalities in the elevated plus maze and startle response, consistent with the idea that late exon 26 ablations may circumvent compensation by Cyt-2 variants. Our findings suggest that ErbB4 Cyt-1 function in vivo is important for modulating DA levels and for regulating behaviors in adult mice [Reference 3].
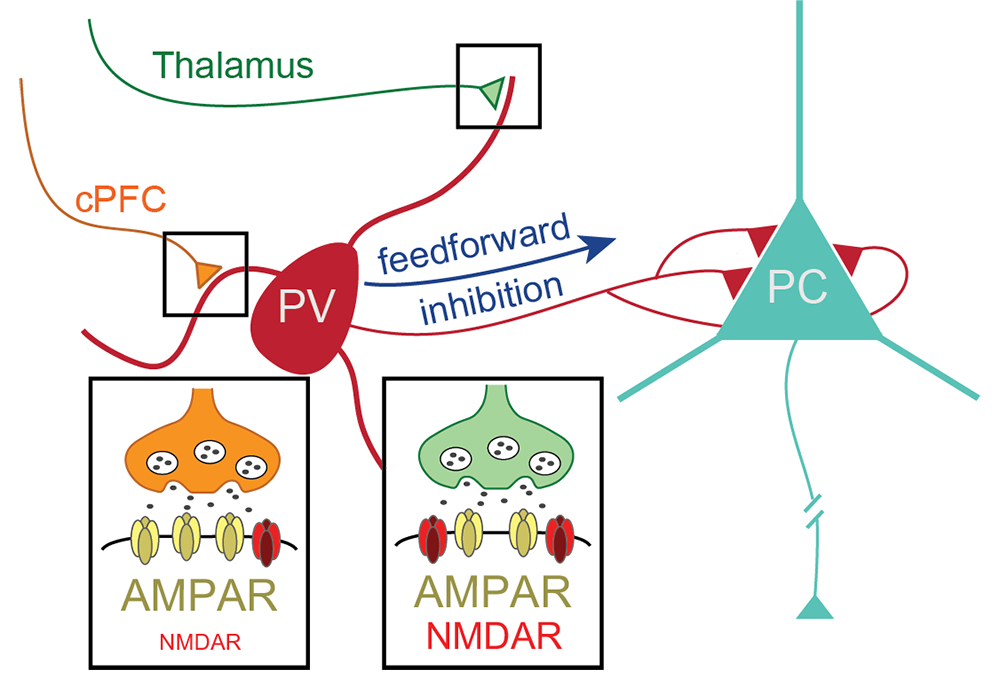
Pathway-specific contribution of parvalbumin interneuron NMDARs to synaptic currents and thalamocortical feedforward inhibition
Despite the importance of understanding how glutamatergic inputs onto PV+ interneurons affect network activity, behavior, and disease, there continues to be controversy as to whether both AMPA and NMDA glutamate receptors or only AMPARs contribute to their excitatory drive. Using a combination of molecular, electrophysiological, and optogenetic approaches, in combination with selective gene-targeting techniques in PV+ interneurons, we resolved this long-standing controversy. We found that nearly 100% of PV+ interneurons in adult medial PFC express the NMDAR subunits GluN1 and GluN2B, and that they have functional NMDARs. With selective optogenetic stimulation of corticocortical or thalamocortical inputs onto PV+ interneurons in the PFC, we found that the relative synaptic NMDAR contribution to excitatory post-synaptic currents is pathway-specific, with NMDARs contributing more at thalamocortical synapses. The pathway-specific contribution of NMDARs to PV+ interneuron excitation is likely to explain earlier conflicting reports suggesting the absence of functional synaptic NMDARs in this GABAergic interneuron subtype in the adult PFC. We then went on to determine whether NMDAR currents in PV+ interneurons contribute significantly to PFC neuronal network activity. Indeed, we found that PV+ interneuron NMDARs contribute to thalamus-mediated feedforward inhibition in PFC circuits, suggesting molecular and circuit-based mechanisms for cognitive impairment under conditions of reduced NMDAR function (Figure 5). The findings represent an important conceptual advance, which has major implications for understanding the pathogenesis of psychiatric disorders [Reference 5].
Figure 5. ProNRG2 clustering at ER-PM junctions is mediated by VAP binding and regulated by phosphorylation
Inhibitory fast-spiking GABAergic PV+ interneurons (red) in adult mouse PFC receive excitatory glutamatergic inputs from the contralateral PFC (cPFC; orange) and the ipsilateral thalamus (green). Optogenetic stimulation of either cPFC or thalamocortical inputs onto PV+ interneurons indicate that NMDARs contribute more to the size and shape of excitatory synaptic currents at thalamic than at cPFC synapses. By selectively knocking out NMDAR expression in adult inhibitory PV+ interneurons, we found that expression of these receptors at thalamic synapses of PFC PV+ interneurons are required for feedforward inhibition of pyramidal cells (PC).
Additional Funding
- NICHD DIR Director's Investigator Award (RRC# D-14-07). PI: Andres Buonanno; Co-PI: Juan Bonifacino. “Exploring the functional role of Neuregulin isoform diversity in CNS”
- Center for Compulsive Behaviors Fellowship Award
Publications
- Ahmad T, Vullhorst D, Chaudhuri R, Guardia CM, Chaudhary N, Karavanova I, Bonifacino JS, Buonanno A. Transcytosis and trans-synaptic retention by postsynaptic ErbB4 underlie axonal accumulation of NRG3. J Cell Biol 2022 221:e202110167.
- Skirzewski M, Cronin ME, Murphy R, Fobbs W, Kravitz AV, Buonanno A. ErbB4 null mice display altered mesocorticolimbic and nigrostriatal dopamine levels as well as deficits in cognitive and motivational behaviors. eNeuro 2020 7(3):0395–19.2020.
- Erben L, Welday JP, Cronin ME, Murphy R, Skirzewski M, Vullhorst D, Carroll SL, Buonanno A. Developmental, neurochemical, and behavioral analyses of ErbB4 Cyt-1 knockout mice. J Neurochem 2022 161:435–452.
- Erben L, Welday JP, Murphy R, Buonanno A. Toxic and phenotypic effects of AAV_Cre used to transduce mesencephalic dopaminergic neurons. Int J Mol Sci 2022 23:9462.
- Lewis EM, Spence HE, Akella N, Buonanno A. Pathway-specific contribution of parvalbumin interneuron NMDARs to synaptic currents and thalamocortical feedforward inhibition. Mol Psychiatry 2022 27: 5124–5134.
Collaborators
- Tanveer Ahmad, PhD, Multidisciplinary Centre for Advance Research and Studies, Jamia Millia Islamia, New Delhi, India
- Juan Bonifacino, PhD, Section on Intracellular Trafficking, NICHD, Bethesda, MD
- Steve Carroll, MD, PhD, Medical University of South Carolina, Charleston, SC
- Carlos Guardia, PhD, Section on Intracellular Trafficking, NICHD, Bethesda, MD
- Lex Kravitz, PhD, Washington University Medical School in St. Louis, St. Louis, MO
- Miguel Skirzewski, PhD, University of Western Ontario, London, Canada
- Jung-Hwa Tao-Cheng, PhD, EM Facility, NINDS, Bethesda, MD
Contact
For more information, email buonanno@mail.nih.gov or visit https://smn.nichd.nih.gov.