Hippocampal Interneurons and Their Role in the Control of Network Excitability

- Chris J. McBain, PhD, Head, Section on Cellular and Synaptic Physiology
- Ramesh Chittajulla, PhD, Staff Scientist
- Kenneth Pelkey, PhD, Staff Scientist
- Adam Caccavano, PhD, Postdoctoral Fellow
- June Hoan Kim, PhD, Postdoctoral Fellow
- Vivek Mahadevan, PhD, Postdoctoral Fellow
- Geoffrey Vargish, PhD, Postdoctoral Fellow
- Steven Hunt, BS, Biologist
- Xiaoqing Yuan, MSc, Biologist
- Connie Mackenzie-Gray Scott, BSc, Predoctoral Fellow
- Anna Vlachos, BSc, Postbaccalaureate Fellow
Cortical and hippocampal GABAergic inhibitory interneurons (INs) are ‘tailor-made’ to control cellular and network excitability by providing synaptic and extrasynaptic input to their downstream targets via GABAA and GABAB receptors. The axons of this diverse cell population make local, short-range projections (although some subpopulations project their axons over considerable distances) and release the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) onto a variety of targets. Distinct cohorts of INs regulate sub- and supra-threshold intrinsic conductances, regulate Na+- and Ca2+-dependent action-potential generation, modulate synaptic transmission and plasticity, and pace both local and long-range large-scale synchronous oscillatory activity. An increasing appreciation of the roles played by INs in several neural-circuit disorders, such as epilepsy, stroke, Alzheimer’s disease, and schizophrenia, has seen this important cell type take center stage in cortical circuit research. With almost 30 years of interest in this cell type, the main objectives of the lab have been to understand: (1) the developmental trajectories taken by specific cohorts of INs as they populate the nascent hippocampus and cortex; (2) how ionic and synaptic mechanisms regulate the activity of both local-circuit GABAergic INs and principal neurons (PN) at the level of small, well defined networks; and (3) how perturbations in their function alter the cortical network in several neural-circuit disorders. To this end, we use a variety of electrophysiological, imaging, optogenetic, immunohistochemical, biochemical, molecular, and genetic approaches with both wild-type and transgenic animals.
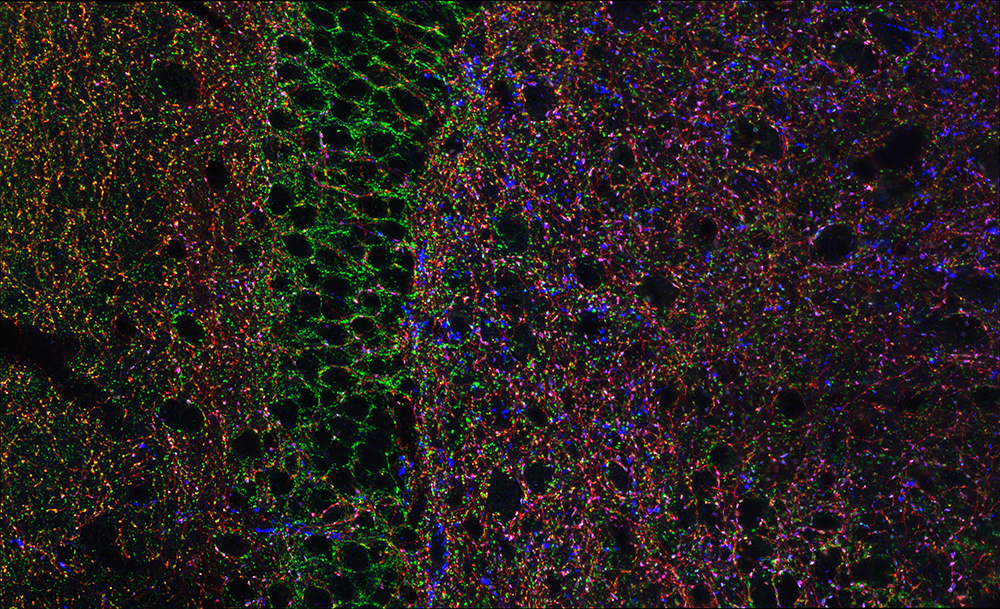
Figure 1. Hippocampal GABAergic terminals
Three major types of GABAergic terminal are shown in the hippocampal dentate gyrus (red, blue, and green punch). The black negative space shows the cell bodies of principal cells innervated by the GABAergic terminals.
NMDARs drive the expression of neuropsychiatric-disorder risk genes within GABAergic interneuron subtypes in the juvenile brain.
Medial ganglionic eminence (MGE)–derived parvalbumin (PV)+, somatostatin (SST)+ and neurogliaform (NGFC)–type cortical and hippocampal interneurons have distinct molecular, anatomical, and physiological properties. However, the molecular mechanisms regulating their maturation remain poorly understood. Via single-cell transcriptomics, we showed that the obligate NMDA–type glutamate receptor (NMDAR) subunit gene Grin1 mediates transcriptional regulation of gene expression in specific subtypes of MGE–derived interneurons, leading to altered subtype abundances. Notably, MGE–specific early developmental Grin1 loss results in a broad downregulation of diverse transcriptional, synaptogenic, and membrane-excitability regulatory programs in the juvenile brain. These widespread gene-expression abnormalities mirror aberrations that are typically associated with neuro-developmental disorders. Our study hence provided a road map for the systematic examination of NMDAR signaling in interneuron subtypes, revealing potential MGE–specific genetic targets that could instruct future therapies of psychiatric disorders.
NPTX2 loss-of-function and schizophrenia
Memory loss in Alzheimer's disease (AD) is attributed to pervasive weakening and loss of synapses. We presented findings supporting a special role for excitatory synapses connecting pyramidal neurons of the hippocampus and cortex with fast-spiking parvalbumin (PV) interneurons, which control network excitability and rhythmicity. Excitatory synapses on PV interneurons are dependent on the AMPA receptor subunit GluA4, which is regulated by presynaptic expression of the synaptogenic immediate early gene NPTX2 by pyramidal neurons. In a mouse model of AD amyloidosis, Nptx2–/– results in reduced GluA4 expression, disrupted rhythmicity, and increased pyramidal neuron excitability. Postmortem human AD cortex shows profound reductions in NPTX2 and coordinate reductions in GluA4. NPTX2 in human CSF is reduced in subjects with AD and shows robust correlations with cognitive performance and hippocampal volume. The findings implicate failure of adaptive control of pyramidal neuron–PV circuits as a pathophysiological mechanism contributing to cognitive failure in AD.
Resilient hippocampal gamma rhythmogenesis and parvalbumin-expressing interneuron function before and after plaque burden in 5xFAD Alzheimer’s disease (AD) model
Recent studies have implicated impaired parvalbumin fast-spiking interneuron (PVIN) function as a precipitating factor underlying abnormalities in network synchrony, oscillatory rhythms, and cognition associated with AD. However, a complete developmental investigation into potential gamma deficits, induced by commonly used carbachol or kainate in ex vivo slice preparations, within AD model mice is lacking. We examined gamma oscillations, using field recordings in acute hippocampal slices from 5xFAD (which carry five familial AD mutations) and in control mice, through the period of developing pathology, starting at three months of age, when there is minimal plaque presence in the hippocampus, through to 12 or more months of age, when plaque burden is high. In addition, we examined PVIN participation in gamma rhythms, using targeted cell-attached recordings of genetically reported PVINs, in both wild-type and mutant mice. In parallel, we compared a developmental immunohistochemical characterization, probing the PVIN–associated expression of PV and perineuronal nets (PNNs), between control and 5xFAD mice. Remarkably, this comprehensive longitudinal evaluation failed to reveal any obvious correlations between PVIN deficits (electrical and molecular), circuit rhythmogenesis (gamma frequency and power), and Aβ deposits/plaque formation. By six to 12 months, 5xFAD animals exhibit extensive plaque formation throughout the hippocampus. However, a deficit in gamma oscillatory power was only evident in the oldest 5xFAD animals (over 12 months), and only when using kainate, and not carbachol, to induce the oscillations. We found no difference in PV firing or phase preference during kainate-induced oscillations in younger or older 5xFAD mice compared with control, and a reduction of PV and PNNs only in the oldest 5xFAD mice. The lack of a clear relationship between PVIN function, network rhythmicity, and plaque formation in our study highlights an unexpected resilience in PVIN function in the face of extensive plaque pathology associated with this model, calling into question the presumptive link between PVIN pathology and AD progression.
A versatile viral toolkit for functional discovery in the nervous system
The ability to precisely control transgene expression is essential for basic research and clinical applications. Adeno-associated viruses (AAVs) are non-pathogenic and can be used to drive stable expression in virtually any tissue, cell type, or species, but their limited genomic payload results in a trade-off between the transgenes that can be incorporated and the complexity of the regulatory elements controlling their expression. Resolving these competing imperatives in complex experiments inevitably results in compromises. We assembled an optimized viral toolkit (VTK) that addresses these limitations and allows for efficient combinatorial targeting of cell types. Moreover, their modular design explicitly enables further refinements. We achieved this in compact vectors by integrating structural improvements of AAV vectors with innovative molecular tools. We illustrated the potential of this approach through a systematic demonstration of their utility for targeting cell types and querying their biology, using a wide array of genetically encoded tools.
Additional Funding
- Connie Mackenzie Gray-Scott was funded by an NIH-Wellcome Trust Graduate Student award.
Publications
- Mahadevan V, Mitra A, Zhang Y, Yuan X, Peltekian A, Chittajallu R, Esnault C, Maric D, Rhodes C, Pelkey KA, Dale R, Petros T, McBain CJ. NMDARs drive the expression of neuropsychiatric disorder risk genes within GABAergic interneuron subtypes in the juvenile brain. Front Mol Neurosci 2021 14:712609.
- Xiao MF, Roh SE, Zhou J, Lucey B, Craig MT, Hays L, Jia M, Chien CC, Xu D, Zhou W, Talbot C, Weinberg D, Sawa A, Pelkey KA, McBain CJ, Savonenko A, Worley PF. A biomarker-authenticated model of schizophrenia implicating NPTX2 loss of function. Sci Adv 2021 7(48):eabf6935.
- Mackenzie-Gray Scott CA, Pelkey KA, Caccavano A, Abebe D, Lai M, Black KN, Brown N, Trevelyan AJ, McBain CJ. Resilient hippocampal gamma rhythmogenesis and parvalbumin-expressing interneuron function before and after plaque burden in 5xFAD Alzheimer’s disease model. Front Synaptic Neurosci 2022 14:857608.
- Pouchelon G, Vergara J, McMahon J, Gorissen B. Lin J, Vormstein-Schneider D, Niehaus JL, Burbridge T, Wester JC, Sherer M, Fernandez-Otero M, Allaway K, Pelkey K, Chittajallu R, McBain CJ, Fan M, Nasse J, Wildenberg J, Fishell G, Dimidschstein J. A versatile viral toolkit for functional discovery in the nervous system. Cell Rep Methods 2022 2:100225.
Collaborators
- Jordan Dimidschstein, PhD, The Broad Institute, Cambridge, MA
- Gordon Fishell, PhD, The Broad Institute, Cambridge, MA
- Timothy J. Petros, PhD, Unit on Cellular and Molecular Neurodevelopment, NICHD, Bethesda, MD
- Andrew Trevelyan, DPhil, MBBCh, MA, Newcastle University, Newcastle, United Kingdom
- Paul F. Worley, MD, Johns Hopkins University School of Medicine, Baltimore, MD
- Kareem Zaghloul, MD, PhD, Functional and Restorative Neurosurgery Unit, NINDS, Bethesda, MD
Contact
For more information, email mcbainc@mail.nih.gov or visit https://www.nichd.nih.gov/research/atNICHD/Investigators/mcbain.