Virulence Mechanisms of Microbial Pathogens

- Matthias Machner, PhD, Head, Section on Microbial Pathogenesis
- Devanand Bondage, PhD, Visiting Fellow
- Katherine Bonnington, PhD, Visiting Fellow
- Candice Chang, PhD, Visiting Fellow
- Nicole Ellis, PhD, Visiting Fellow
- Stephanie Lehman, PhD, Visiting Fellow
- Xiao Li, PhD, Visiting Fellow
- Sabrina Bouchard, BS, Postbaccalaureate Student
- Jacob Olondo-Kuba, BS, Postbaccalaureate Student
Our main research goal is to define mechanisms by which pathogenic bacteria subvert the human host defense and cause disease. In parallel, we investigate whether these mechanisms may be manipulated for preventative and/or therapeutic purposes. As a model organism, we use the bacterium Legionella pneumophila, the causative agent of a potentially fatal respiratory infection known as Legionnaires' disease. According to the CDC (Centers for Disease Control), the number of Legionnaires' disease cases in the U.S. has risen more than four-fold over the past 15 years, making L. pneumophila an emerging pathogen of increasing relevance. Contrary to what its name may imply, Legionnaires’ disease occurs in individuals of all ages, including children who receive respiratory therapy, newborns who had recently undergone surgery or under-water birth, and children who are immune-compromised. We are committed to an in-depth analysis of the mechanisms that allow L. pneumophila to exploit the human host and cause disease. Insights gained from our studies will ultimately improve our ability to diagnose, prevent, and fight Legionnaires’ disease and related illnesses, thereby contributing to the success of NICHD’s mission.
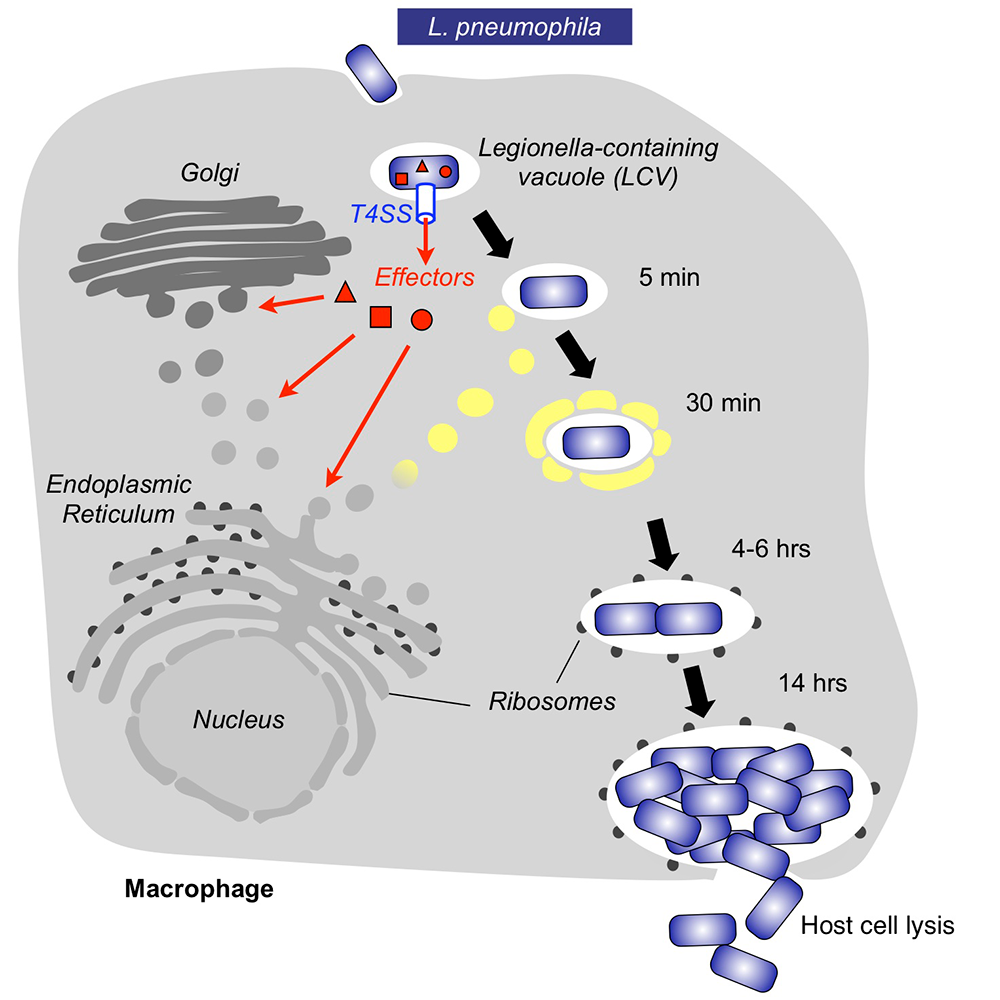
Within freshwater environments, L. pneumophila exists as an intracellular parasite of single-cell organisms known as amoeba. Upon inhalation of contaminated water droplets, L. pneumophila enters the lung and is phagocytosed (taken up) by specialized immune cells known as alveolar macrophages (Figure 1). Instead of being degraded by these cells, the pathogen establishes a protective membrane compartment, the Legionella-containing vacuole (LCV). Within this intravacuolar niche, L. pneumophila can replicate to high numbers before killing the host cell and infecting neighboring cells.
The virulence of L. pneumophila relies on the activity of close to 300 proteins, or effectors, that are delivered into the host cytosol by a specialized translocation apparatus called the Dot/Icm type IV secretion system (T4SS) (Figure 1). L. pneumophila mutants with a non-functional T4SS are degraded by macrophages, underscoring the importance of the translocated effectors for host-cell manipulation and bacterial virulence.
Our main objective is to obtain detailed mechanistic insight into L. pneumophila effectors by investigating their biological role at molecular, cellular, and structural levels. Knowledge obtained from these studies can help in the development of novel therapeutics aimed at treating or preventing Legionnaires' disease and related illnesses.
Figure 1. Intracellular replication cycle of Legionella pneumophila
Upon uptake by a macrophage, L. pneumophila delivers a large number of effector proteins (red) through the Dot/Icm type IV secretion system (T4SS) into the host cytosol. The effectors manipulate signaling and trafficking pathways in order to establish conditions favorable for L. pneumophila growth. Eventually, the host cell is lyzed, and L. pneumophila bacteria infect neighboring cells.
Comprehensive phenotypic screening strategy to identify modulators of cargo translocation by the bacterial type IVB secretion system
Multi-drug-resistant pathogens are an emerging threat to human health. Because conventional antibiotics target not only the pathogen but also eradicate beneficial microbiota, they often cause additional clinical complications. Thus, there is an urgent need for the development of “smarter” therapeutics that selectively target pathogens without affecting beneficial commensals. The bacterial type IV secretion system (T4SS) is essential for the virulence of a variety of pathogens but dispensable for bacterial viability in general and can, however, be considered a pathogen's Achilles heel.
Like many other pathogens, L. pneumophila relies on a T4SS (called Dot/Icm) for colonization and proliferation within a wide range of host cells, including freshwater amoeba in the environment and alveolar macrophages during Legionnaires' pneumonia in humans. L. pneumophila mutants with a non-functional T4SS fail to control trafficking of their LCV and are quickly delivered to lysosomes for degradation, thus underscoring the importance of the Dot/Icm system for Legionella pathogenesis.
We designed an automated high-throughput screening approach for the identification of compounds that interfere with the delivery of a reporter-effector fusion protein from L. pneumophila into RAW264.7 mouse macrophages. Using a fluorescence resonance energy transfer (FRET)–based detection assay in a bacteria/macrophage coculture format, we screened a library of over 18,000 compounds and, upon vetting compound candidates in a variety of in vitro and cell-based secondary screens, isolated several hits that efficiently interfered with biological processes that depend on a functional T4SS, such as intracellular bacterial proliferation or lysosomal avoidance, but had no detectable effect on L. pneumophila growth in culture medium, conditions under which the T4SS is dispensable. Notably, the same hit compounds also attenuated, to varying degrees, effector delivery by the closely related T4SS from Coxiella burnetii, notably without impacting growth of this organism within synthetic media. Together, the results support the idea that interference with T4SS function is a possible therapeutic intervention strategy, and the emerging compounds provide tools to interrogate, at a molecular level, the regulation and dynamics of these virulence-critical translocation machines. Our study represents the first step in our pursuit toward precision medicine by developing pathogen-selective therapeutics capable of treating the infections without causing harm to commensal bacteria.
Figure 2. Compounds that target the T4SS attenuate L. pneumophila intracellular growth.
(A) Schematic overview of the intracellular growth assay with bacterial in green and host cell nuclei in blue.
(B) Growth of Legionella in macrophages is attenuated in the presence of hit compounds but not in untreated control cells.
(C) The inhibition of L. pneumophila growth by select compounds (C1 to C7) is dose-dependent.
VpdC is a ubiquitin-activated phospholipase effector that regulates Legionella vacuole expansion during infection.
Phospholipids are a major component of membranes. They are composed of two lipophilic fatty acids, a glycerol backbone, and a hydrophilic head with a phosphate group that can be esterified to other biomolecules. As a result of their amphipathic character, phospholipids have the tendency to form bilayers that allow lateral fluidity while providing a diffusion barrier with mechanical strength against rupture. L. pneumophila exploits those features by hiding inside a membrane-enclosed phagosomal compartment that provides protection from immune detection by the host. At the same time, the phagosomal membrane confines L. pneumophila to a tight space that needs to be gradually expanded during intracellular replication to accommodate the growing number of offspring. How vacuole expansion is controlled by pathogens has remained unclear.
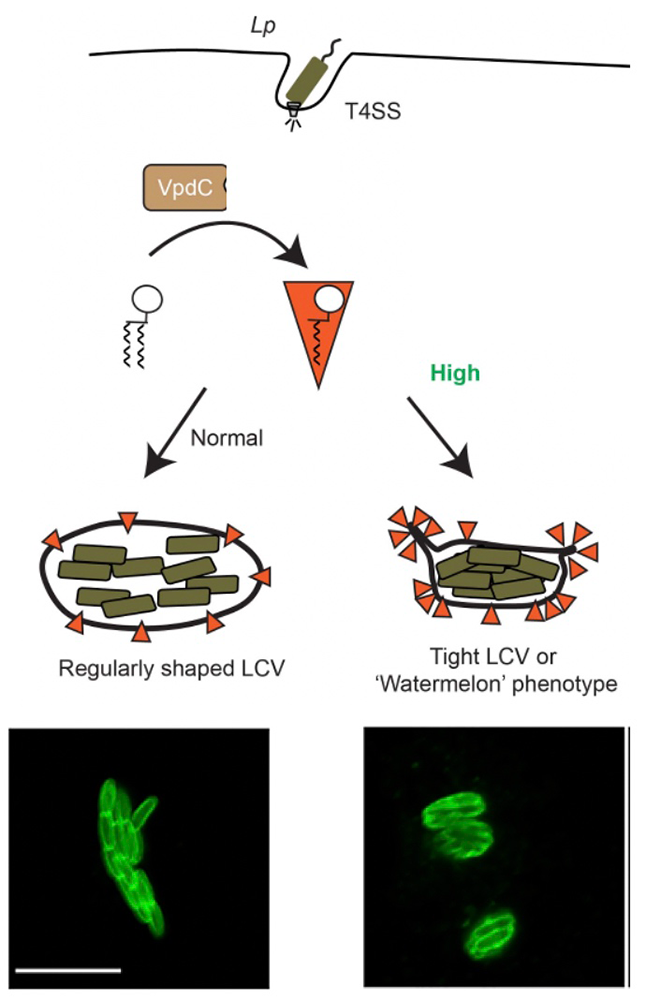
We discovered that the phospholipase effector VpdC plays a major role in this process. Phospholipases are enzymes that hydrolyze phospholipids to generate free fatty acids and lysophospholipids. Owing to their inverted-cone shape, lysophospholipids can affect membrane curvature and thus profoundly influence membrane fusion. We discovered that the phospholipase effector VpdC generates lysophospholipids within the phagosomal membrane surrounding L. pneumophila. While moderate levels of lysophospholipids can promote membrane fusion, elevated levels obstruct fusion. Consequently, we found that VpdC overproduction blocked membrane expansion of Legionella-containing vacuoles, trapping the bacteria inside a spatially confined compartment and attenuating their virulence. Together, our findings not only demonstrate an important role for bacterial phospholipases in vacuolar expansion, but also suggest that exploiting lysophospholipids for their membrane-bending capability might be a common strategy among pathogens to control membrane dynamics.
Figure 3. Lysophospholipids play an important role in regulating vacuole expansion during Legionella growth.
Upon infection of human macrophages, the effector protein VpdC from Legionella (Lp) generates moderate levels of lysophospholipids (orange triangle) within the membrane of its vacuole (LCV) to promote expansion. Overproduction of lysophospholipids prevents membrane acquisition and expansion of the LCV, trapping the bacteria (labeled green in the micrograph) within spatially confined vacuoles and reducing virulence. Scale bar, 5 μm.
Additional Funding
- NICHD Early Career Awards FY22 (to Yuen Yan Chang)
- NICHD Early Career Awards FY22 (to Stephanie Lehman)
Publications
- Li X, Anderson DE, Chang Y-Y, Jarnik M, Machner MP. VpdC is a ubiquitin-activated phospholipase effector that regulates Legionella vacuole expansion during infection. Proc Natl Acad Sci U S A 2022 119(48):e2209149119.
- Cheng E, Dorjsuren D, Lehman S, Larson CL, Titus SA, Sun H, Zakharov A, Rai G, Heinzen RA, Simeonov A, Machner MP. A comprehensive phenotypic screening strategy to identify modulators of cargo translocation by the bacterial Type IVB secretion system. mBio 2022 13:e0024022.
Collaborators
- Eric D. Anderson, PhD, Advanced Mass Spectrometry Core, NIDDK, Bethesda, MD
- Robert Heinzen, PhD, Coxiella Pathogenesis Section, Rocky Mountain Laboratories, NIAID, Hamilton, MT
- Michal Jarnik, PhD, Section on Intracellular Protein Trafficking, NICHD, Bethesda, MD
- Anton Simeonov, PhD, Division of Pre-Clinical Innovation, NCATS, Bethesda, MD
- Chad Williamson, PhD, Advanced Microscope Facility, NICHD, Bethesda, MD
Contact
For more information, email machnerm@mail.nih.gov or visit https://machnerlab.nichd.nih.gov.