Mechanisms of Disease in Preterm Labor and Complications of Prematurity; Prenatal Diagnosis of Congenital Anomalies

- Roberto Romero, MD, DMedSci, Chief, Perinatology Research Branch
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide, and two-thirds of all preterm births occur after the onset of preterm labor. The cost of prematurity in the United States alone was estimated at $26 billion per year in 2007. Therefore, the important goals are to understand the mechanisms of disease responsible for preterm birth and fetal injury and to improve the prediction and prevention of preterm birth. The Pregnancy Research Branch proposed that preterm parturition is a syndrome caused by multiple pathologic processes (Romero R et al. Science 2014;345:760). The Branch developed methods for the rapid diagnosis of intra-amniotic infection/inflammation and showed that such pathologic processes can be treated successfully. In addition, the current approach to predict and prevent spontaneous preterm birth in clinical obstetrics is based on the work of the Branch (the PREGNANT trial and subsequent meta-analyses). The research team continues to study the physiology of pregnancy and parturition to inform studies of spontaneous preterm labor, and, in particular, the use of high-dimensional, post-genomic tools, such as transcriptomics, proteomics, and the analysis of parturition at single-cell resolution.
Imaging is a powerful instrument for scientific discovery, which has changed the practice of obstetrics and maternal-fetal medicine. The single most important step that has made fetal medicine a discipline is the transformation of the fetus from an invisible to a visible subject through the use of imaging techniques, in particular, ultrasound. This technology has allowed the definition of fetal anatomy, biometry, and growth as well as the study of physiologic parameters, e.g., cardiac function, fetal sleep, and breathing. We use different imaging modalities to examine the diagnosis of anomalies and obstetrical syndromes. Such modalities include ultrasound (2-dimensional, 3-dimensional), magnetic resonance imaging, and optical methods. We have also utilized other imaging techniques to study the human placenta.
Understanding the mechanisms of human term and preterm parturition through transcriptomics
A single-cell atlas of the myometrium in human parturition
Few biological processes as central to the survival of viviparous species are so incompletely understood as parturition. Yet, there is an inadequate understanding of the physiology of normal labor and the pathophysiology of labor disorders, preterm and term. The Branch used bulk transcriptomics to understand the components of the common pathway of parturition, which include myometrial contractility, cervical remodeling, and membrane/decidual activation, and we characterized differentially expressed genes and biological processes enriched during parturition. We leveraged single-cell technology to assemble a human cell atlas of parturition and analyzed the placenta and chorioamniotic membranes. However, the engine of labor is the myometrium, which is inaccessible for study. Therefore, one of our goals has been to determine whether it is possible to monitor cellular activity in the myometrium non-invasively through maternal blood. We performed RNA single-cell sequencing (scRNA-Seq) and generated the first single-cell atlas of the human myometrium during labor as well as the first map of cell type–specific transcriptomic activity modulation. This information provides the foundation for studying labor disorders. Integrating scRNA-Seq information with transcriptomic data derived from bulk analyses of the myometrium, we characterized the contributions of smooth muscle cells and inflammation during labor. Importantly, we showed that myometrium-derived single-cell signatures could be detected and quantitated in maternal blood. The result provides evidence that it is possible to monitor myometrial biology non-invasively by interrogating maternal blood. Indeed, we developed a transcriptomic signature of labor in maternal blood in term and preterm labor.
The proteome of human pregnancy and preterm delivery
The amniotic-fluid proteome in normal pregnancy and impending preterm delivery
We studied the amniotic-fluid proteome to establish a systems-biology approach for pregnancy complications. We first characterized the amniotic fluid proteome and found that about 25% (320/1310) of proteins changed significantly in abundance with gestational age. Intersecting gestational age–modulated proteins and their corresponding mRNAs, previously reported in the maternal blood, identified neutrophil-related protein/mRNA pairs modulated in the same direction. Our observations have implications for the discovery of biomarkers to diagnose obstetrical and fetal disorders.
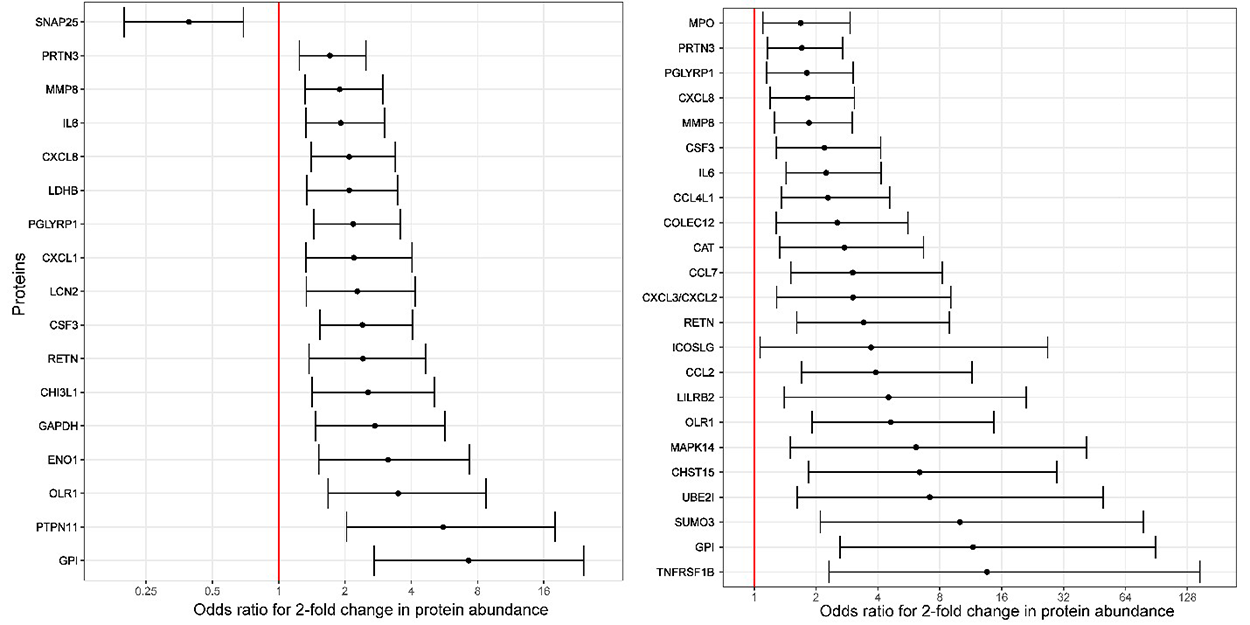
Figure 1. Odds ratios (and 95% confidence intervals) for the association between amniotic fluid protein abundance and imminent preterm delivery among asymptomatic women with a short cervix
Odds ratios are shown for delivery within two weeks (left) and within one week from the amniocentesis (right). The odd ratios are calculated for a two-fold change in protein abundance and are adjusted for cervical length.
A sonographic short cervix, the most powerful predictor of spontaneous preterm birth, is an indication for treatment with vaginal progesterone to prevent preterm birth. However, only a fraction of women respond to progesterone. Therefore, markers are required to determine which patients with a sonographic short cervix will progress to imminent delivery. We conducted a study to identify proteomic signatures of impending preterm delivery in patients with an asymptomatic sonographic short cervix (less than 25 mm) at 16 to 32 weeks of gestation. A combination of four amniotic fluid proteins (CXCL8, SNAP25, PTPN11, and MMP8) had a sensitivity of 79%, with a 10% false-positive rate, for the prediction of delivery within two weeks. This was a substantial improvement over the prediction derived from cervical length alone. We have already developed rapid point-of-care tests for determining CXCL8 and MMP-8 in amniotic fluid. These observations have clinical implications, given the recent evidence generated by our group that intra-amniotic inflammation can be eradicated, leading to prolongation of pregnancy. In addition, the diagnosis of silent intra-amniotic inflammation has implications for the management of the patient presenting with an asymptomatic short cervix.
Novel molecular and cellular treatments to prevent premature labor and fetal injury
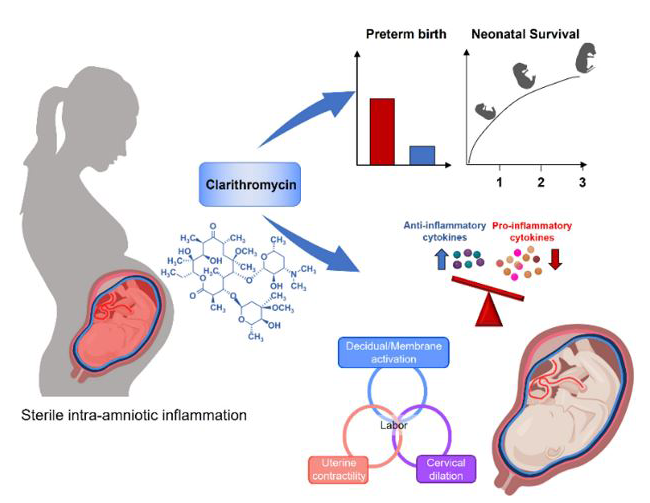
Treatment of sterile intra-amniotic inflammation with clarithromycin prevents preterm delivery and reduces neonatal morbidity through modulation of inflammatory responses in maternal and fetal tissues.
Sterile intra-amniotic inflammation has emerged as the most frequent identifiable etiology of spontaneous preterm labor with intact membranes and cervical insufficiency. Our recent clinical studies demonstrated that successful treatment of sterile intra-amniotic inflammation can be achieved with clarithromycin, an antimicrobial agent with strong anti-inflammatory properties. We conducted several studies to determine the mechanisms by which clarithromycin prolongs gestation and improves neonatal outcomes. Using a previously established animal model of sterile intra-amniotic inflammation generated in our laboratory, we found that clarithromycin prolonged gestation, reduced the rate of preterm birth, and improved neonatal survival. Clarithromycin also exhibited potent anti-inflammatory effects in the placental and fetal tissues, which may contribute to the improved neonatal outcomes. The findings provide mechanistic evidence that clarithromycin can be administered to prevent preterm birth and to improve neonatal survival in the context of sterile intra-amniotic inflammation [Galaz J. et al. BMC Pregnancy Childbirth 2022;22:503].
Figure 2.
Association between SARS-CoV-2 infection severity and pregnancy outcome
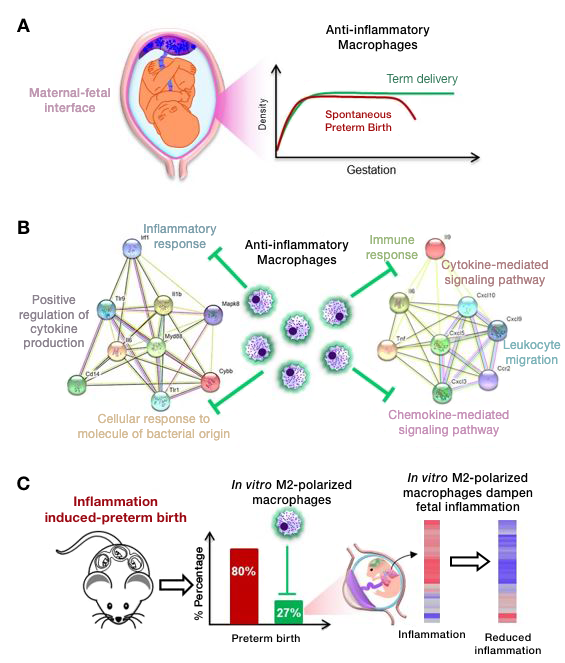
The role of maternal macrophages in the maintenance of pregnancy and the potential use of M2–polarized macrophages as cellular therapy to protect against inflammation-induced premature labor and fetal injury
Macrophages participate in the mechanisms of preterm and term labor by amplifying inflammation; however, there is a considerable body of evidence that a subset of macrophages, typically referred to as M2 macrophages, have powerful anti-inflammatory effects. We undertook a series of experiments to study the role of macrophages in pregnancy and observed that: (1) women in labor had a reduced number of M2–like macrophages at the maternal-fetal interface; 2) depletion of maternal CD11b+ myeloid cells led to preterm labor and adverse neonatal outcomes, which was ameliorated by replacement of wild-type macrophages; (3) adoptive transfer of M2–polarized macrophages in vitro reduced the incidence of preterm birth and improved neonatal survival in a model of intra-amniotic inflammation; and (4) M2–polarized macrophages downregulated the inflammatory response in the fetal brain and lungs (major target organs in the fetal inflammatory response syndrome). The results demonstrate a hitherto unappreciated homeostatic role for macrophages in the physiology of pregnancy and a therapeutic role for M2–polarized macrophages for the treatment of premature labor and of the fetal inflammatory response syndrome.
Figure 3.
Macrophages exert homeostatic actions in pregnancy to protect against preterm birth and fetal inflammatory injury.
Refining the use of progesterone for the prevention of preterm birth
Our previous studies showed that vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix in the mid-trimester and reduces neonatal morbidity. Some professional organizations have proposed that vaginal progesterone should also be used in patients who have a history of preterm birth, regardless of cervical length. Therefore, we conducted two meta-analyses and showed that vaginal progesterone is not efficacious in patients with a prior history of preterm birth or in those without a short cervix, which has immediate implications for clinical practice.
Quantifying calcium changes in the fetal spine using quantitative susceptibility mapping as extracted from STAGE imaging
Even though ultrasound is the standard method to image the fetus, it has limitations, e.g., for characterizing the state of mineralization of the fetal spine. Computer tomography (CT) could be considered an alternative, but it carries the risk of ionizing radiation to the fetus and the mother. Fetal skeletal bone development during pregnancy has been qualitatively described by different imaging modalities, yet there is a paucity of quantitative evidence of bone development as a function of gestational age in the second and third trimesters. Similar to iron, calcium in the bones also induces severe-phase dephasing at longer echo times. However, as opposed to iron, which is paramagnetic with positive susceptibility, calcium is diamagnetic, meaning that it shows a negative susceptibility. Quantitative susceptibility mapping (QSM) has also been introduced as an in vivo non-ionizing imaging alternative to CT from which bone calcification can be monitored and quantified longitudinally over the course of pregnancy. We successfully evaluated the susceptibility of the fetal spine and demonstrated a strongly decreasing susceptibility with gestational age. For the first time, our results have been able to evaluate changes in fetal bone calcification over time.
Figure 4. Average susceptibility (associated with calcium) of the vertebrae plotted as a function of gestational age
The decreasing susceptibility with gestational age indicates that the in vivo theory behind increasing fetal calcium content during fetal development.
The role of the placenta in spontaneous preterm labor and delivery with intact membranes
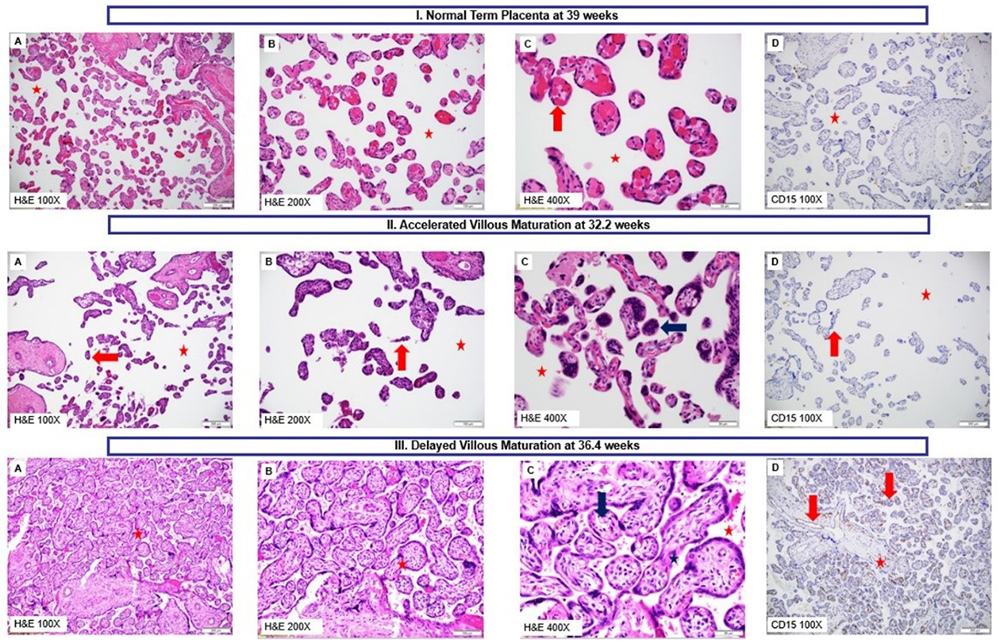
Preterm birth is the leading cause of death in children younger than five years of age. It accounts for 35% of deaths in neonates and affects 10.6% of live births. Infants who survive a preterm birth have a higher rate of long-term morbidity, including neurologic and developmental disabilities, and a shorter life expectancy than infants born full term. Fetal nutrition depends on the blood flow and its circulation on the maternal and fetal sides of the placenta. Therefore, an important pathway whereby the placenta causes non-infection/inflammation-related complications of pregnancy must involve abnormalities of the fetoplacental circulation that impair the exchange function of the placenta. We conducted a study to determine whether placental vascular pathology and impaired placental exchange (resulting from maturational defects) are involved in the etiology of spontaneous preterm labor and delivery in cases without infection/inflammation, i.e., without histologic acute chorioamnionitis. An evaluation was performed for 184 cases of pregnancies resulting in spontaneous preterm labor and delivery (less than 37 weeks of gestation) and for 2,471 controls in uncomplicated pregnancies that delivered fetuses at term (37–42 weeks of gestation). We found that the frequency of maternal blood flow abnormalities, represented pathologically as lesions of maternal vascular malperfusion, was greater in the placentas of patients with preterm labor than in the control group, i.e., 14.1% (26/184) vs. 8.8% (217/2471). Likewise, placental developmental abnormalities, termed as disorders of villous maturation, were more frequent in the group with preterm labor (41.1%; 39/95) than in the control group: delayed villous maturation, 31.6% (30/95) of cases vs. 2.5% (13/519) of controls; accelerated villous maturation, 9.5% (9/95) of cases vs. 0% of controls. We demonstrated that maturational defects of placental villi contributed to unexplained spontaneous preterm labor and delivery of appropriate-for-gestational-age fetuses in the absence of acute inflammatory lesions of the placenta.
Figure 5.
Panel I: Normal Term Placenta (39 weeks). A–C. Normal chorionic villi that are mature and appropriate for gestational age with normal intervillous space (white space; red star); C. Mature terminal villi with arrows pointing towards vasculo-syncytial membranes formed by the apposition of syncytiotrophoblasts with villous capillary endothelium (normal 3–5 per terminal villi); terminal villi with conspicuous capillaries and barely discernible stroma; D. Chorionic villous capillary endothelium negative for CD-15 staining (absence of brown staining). A–C: H&E-100X, 200X, and 400X; D: CD-15-100X
Panel II: Placenta at gestational age 32.2 weeks showing accelerated villous maturation. A–C. Chorionic villi displaying histology resembling that of term villi (see Panel I) with considerably increased intervillous space (white space; red star) for the gestational age; the terminal villi appear slender, hypermature, hypoplastic (red arrows). C. Hypermature villi with villous syncytial knotting (blue arrow). F. CD-15 negative chorionic villous capillary endothelium (absence of brown staining). A–C: H&E-100X, 200X, and 400X; D: CD-15-100X.
Panel III: Placenta at gestational age 36.4 weeks showing delayed villous maturation. A–C. Chorionic villi appear crowded, immature, affecting more than 50% of the placental villous population and resembling the histology of second trimester villi. Intervillous space is considerably diminished to virtually absent (red star). The villi display more stroma (blue star), centralized vessels (red arrows), and paucity of vasculo-syncytial membranes, as compared with a normal pregnancy (see Panel I). D. CD15–positive (brown staining) capillary endothelium in mature intermediate chorionic villi and stem villi. A–C: H&E-100X, 200X, and 400X; D: CD-15-100X.
Publications
- Motomura K, Romero R, Plazyo O, Garcia-Flores V, Gershater M, Galaz J, Miller D, Gomez-Lopez N. The alarmin S100A12 causes sterile inflammation of the human chorioamniotic membranes as well as preterm birth and neonatal mortality in mice. Biol Reprod 2021 1494–1509.
- Garcia-Flores V, Romero R, Xu Y, Theis KR, Arenas-Hernandez M, Miller D, Peyvandipour A, Bhatti G, Galaz J, Gershater M, Levenson D, Pusod E, Tao L, Kracht D, Florova V, Leng Y, Motomura K, Para R, Faucett M, Hsu CD, Zhang G, Tarca AL, Pique-Regi R, Gomez-Lopez N. Maternal-fetal immune responses in pregnant women infected with SARS-CoV-2. Nat Commun 2022 1–20.
- Pique-Regi R, Romero R, Garcia-Flores V, Peyvandipour A, Tarca AL, Pusod E, Galaz J, Miller D, Bhatti G, Para R, Kanninen T, Hadaya O, Paredes C, Motomura K, Johnson JR, Jung E, Hsu CD, Berry SM, Gomez-Lopez N. A single-cell atlas of the myometrium in human parturition. JCI Insight 2022 1–21.
- Oh KJ, Romero R, Kim HJ, Jung E, Gotsch F, Suksai M, Yoon BH. A role for intraamniotic inflammation in threatened midtrimester miscarriage. Am J Obstet Gynecol 2022 1.e1–1.e13.
- Romero R, Jung E, Chaiworapongsa T, Erez O, Gudicha DW, Kim YM, Kim JS, Kim B, Kusanovic JP, Gotsch F, Taran AB, Yoon BH, Hassan SS, Hsu CD, Chaemsaithong P, Gomez-Lopez N, Yeo L, Kim CJ, Tarca AL. Toward a new taxonomy of obstetrical disease: improved performance of maternal blood biomarkers for the great obstetrical syndromes when classified according to placental pathology. Am J Obstet Gynecol 2022 615.e1–615.e25.
- Purandare N, Kunji Y, Xi Y, Romero R, Gomez-Lopez N, Fribley A, Grossman LI, Aras S. Lipopolysaccharide induces placental mitochondrial dysfunction in murine and human systems by reducing MNRR1 levels via a TLR4-independent pathway. iScience 2022 25:105342.
Collaborators
- Tinnakorn Chaiworapongsa, MD, Wayne State University School of Medicine, Detroit, MI
- Agustin Conde-Agudelo, MD, Wayne State University School of Medicine, Detroit, MI
- Mark Haacke, PhD, Wayne State University School of Medicine, Detroit, MI
- Leonid Margolis, PhD, Section on Intercellular Interactions, NICHD, Bethesda, MD
- Adi L. Tarca, PhD, Wayne State University, Detroit Medical Center, Detroit, MI
- Lami Yeo, MD, Wayne State University School of Medicine, Detroit, MI
- Bo Hyun Yoon, MD, PhD, Seoul National University, Seoul, Korea
Contact
For more information, email romeror@mail.nih.gov or visit https://irp.nih.gov/pi/roberto-romero.