Biophysics of Large Membrane Channels

- Sergey M. Bezrukov, PhD, DSci, Head, Section on Molecular Transport
- Tatiana K. Rostovtseva, PhD, Associate Scientist
- Motahareh Ghahari Larimi, PhD, Visiting Fellow
- Megha Rajendran, PhD, Visiting Fellow
- Kaitlin Abrantes, BS, Intramural Research Training Award Fellow
Our section studies the mitochondrial and bacterial membrane proteins that form “large” beta-barrel channels, which are the gateways of metabolite exchange and components of many toxins recognized as novel drug targets. Currently, we mainly focus on the metabolite-transporting channel VDAC of the outer mitochondrial membrane, but other beta-barrel channels such as MspA (major outer-membrane porin from Mycobacterium smegmatis), alpha-hemolysin (toxin from Staphylococcus aureus), OmpF (general bacterial porin from Escherichia coli), LamB (sugar-specific bacterial porin from Escherichia coli), OprF (porin from Pseudomonas aeruginosa), translocation pores of B. anthracis (PA63), and C. botulinum (C2IIa) and C. perfringens (Ib) binary toxins are also within our interest. Despite having drastically different biological functions, these channels have much in common regarding their biophysical characteristics, so that comparative analysis gives a better understanding of the underlying physical principles. We also use gramicidin A (linear pentadecapeptide from Bacillus brevis) as a molecular sensor to probe the surface charge and mechanical properties of membranes.
We investigate the channels under precisely controlled conditions, first isolating the corresponding channel-forming proteins from their host organisms or refolding recombinant proteins from inclusion bodies, purifying, and then reconstituting them into planar lipid membranes for electrophysiological examination. Our emphasis on single-channel experiments allows us to study transport processes at a single-molecule level. The obtained findings pave the way for the rational design of new strategies and pharmacological approaches to effectively correct the deviant molecular interactions associated with pathology in disease and development.
Voltage-activated complexation of alpha-synuclein with beta-barrel channels and its inhibition as a potential therapeutic target for Parkinson’s disease treatment
Voltage-activated protein complexation is the process by which a transmembrane potential drives complex formation between a membrane-embedded channel and a soluble or membrane-peripheral target protein (Figure 1). Metabolite and calcium flux across the mitochondrial outer membrane was shown to be regulated by voltage-activated complexation of the voltage-dependent anion channel (VDAC) and either dimeric tubulin or alpha-synuclein (αSyn). However, the roles played by the VDAC’s characteristic attributes—its anion selectivity and voltage-gating behavior—have remained unclear. This past year, we conducted a comparative analysis of in vitro measurements of voltage-activated complexation of αSyn with three well-characterized beta-barrel channels, VDAC, MspA, and alpha-hemolysin, that differ widely in their organism of origin, structure, geometry, charge-density distribution, and voltage-gating behavior. The voltage dependences of the complexation dynamics for the different channels were observed to differ quantitatively but to have similar qualitative features. In each case, energy-landscape modeling describes the complexation dynamics in a manner consistent with the known properties of the individual channels, while voltage gating does not appear to play any role. The reaction free energy landscapes thus calculated reveal a common physical mechanism of complexation for all three channels, together with a non-trivial dependence of the complex stability on the surface density of αSyn.
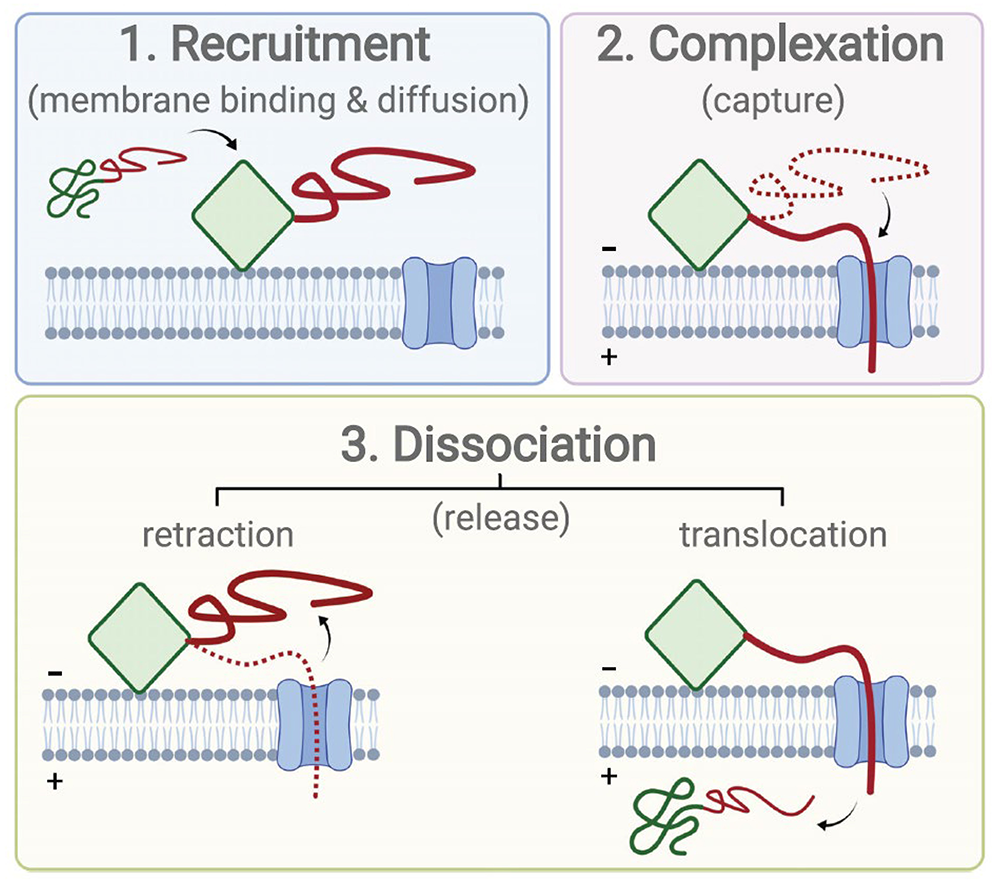
Figure 1. Voltage-activated complexation between a membrane-embedded channel and a peripheral protein
The peripheral protein is shown with two domains: a membrane-binding domain (green), which can be structured or not, and a polyanionic, disordered domain (red). Complexation begins with recruitment of the peripheral protein to the channel-containing membrane and diffusion along the membrane surface to the vicinity of the channel. Direct capture from solution is also possible, in which case the membrane-binding domain is not necessary, but is much less efficient because of the increased dimensionality of the diffusion space. The complex is formed when the polyanionic domain of the peripheral protein is captured into the channel by the transmembrane potential. Finally, the peripheral protein is released either by retraction from the channel or translocation of the entire (unraveled) polypeptide.
It is well recognized that involvement of αSyn in Parkinson’s disease (PD) is complicated and difficult to trace on cellular and molecular levels. Recently, we established that αSyn can regulate mitochondrial function by voltage-activated complexation with the VDAC, as described above. When complexed with αSyn, the VDAC pore is partially blocked, reducing the transport of ATP/ADP and other metabolites though the mitochondrial outer membrane. Further, αSyn can translocate into the mitochondria through the VDAC, where it interferes with mitochondrial respiration. Recruitment of αSyn to the VDAC–containing lipid membrane appears to be a crucial prerequisite for both the blockage and translocation processes. This year, we studied an inhibitory effect of HK2p, a small membrane-binding peptide from the mitochondria-targeting N-terminus of hexokinase 2, on αSyn membrane binding, and hence on αSyn complex formation with the VDAC and translocation through it. In electrophysiology experiments, the addition of HK2p at micromolar concentrations to the same side of the membrane as αSyn results in a dramatic reduction in the frequency of blockage events in a concentration-dependent manner, reporting on complexation inhibition. Using two complementary methods of measuring protein-membrane binding, bilayer overtone analysis and fluorescence-correlation spectroscopy, we found that HK2p induces detachment of αSyn from lipid membranes. Experiments with HeLa cells, using a proximity ligation assay, confirmed that HK2p impedes αSyn entry into mitochondria. Our results demonstrate that it is possible to regulate αSyn–VDAC complexation by a rationally designed peptide, thus suggesting new avenues in the search for peptide therapeutics to alleviate αSyn mitochondrial toxicity in PD and other synucleinopathies.
The single residue K12 governs the exceptional voltage sensitivity of VDAC gating.
The VDAC is the most abundant protein in the mitochondrial outer membrane (MOM) and is the primary conduit for ions and water-soluble metabolites, such as ATP and ADP, to cross the MOM. As such, the VDAC plays a central role in the regulation of MOM permeability and mitochondrial metabolism, and in communication between mitochondria and the rest of the cell. The VDAC responds to a transmembrane potential by “gating,” i.e., transitioning to one of a variety of low-conducting states of unknown structure. The gated state results in nearly complete suppression of multivalent mitochondrial metabolite (such as ATP and ADP) transport, while enhancing calcium transport. Voltage gating is a common property of beta-barrel channels and has been observed in bacterial outer-membrane porins as well as in anthrax, aerolysin, and alpha-hemolysin toxins, but VDAC gating is anomalously sensitive to transmembrane potential. This past year, we showed that a single residue in the pore interior, K12, is responsible for most of the VDAC’s voltage sensitivity.
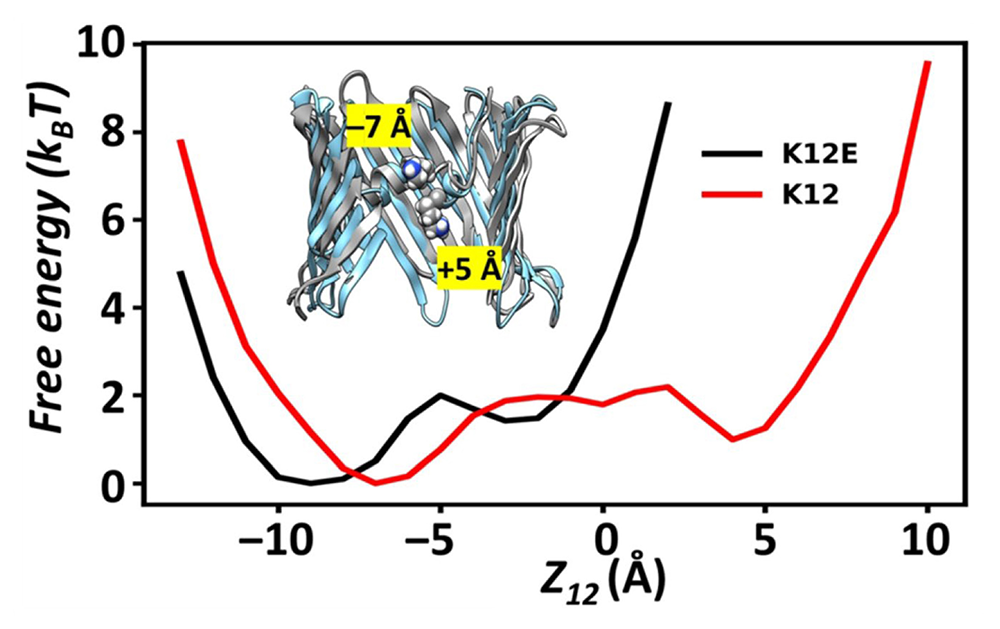
Using the analysis of over 40 microseconds of atomistic molecular dynamics (MD) simulations, we explored correlations between motions of charged residues inside the VDAC pore and geometric deformations of the beta-barrel. Residue K12 is bistable (Figure 2, red curve); its motions between two widely separated positions along the pore axis enhance the fluctuations of the beta-barrel and augment the likelihood of gating. Single-channel electrophysiology of various K12 mutants reveals a dramatic reduction in voltage-induced gating transitions. The crystal structure of the K12E mutant at a resolution of 2.6 Å indicates a similar architecture of the K12E mutant to the wild type; however, 60 microseconds of atomistic MD simulations using the K12E mutant showed restricted motion of residue 12 (Figure 2, black curve), resulting from enhanced connectivity with neighboring residues, and diminished amplitude of barrel motions. We thus conclude that beta-barrel fluctuations, governed particularly by residue K12, drive VDAC gating transitions.
Figure 2. Atomistic MD simulations of the VDAC
1D Free Energy Profile of residue 12 in the wild-type (K12) or mutant (K12E) VDAC along the Z-axis of the channel. The coordinate z12 denotes the z-coordinates of the nitrogen atom of K12 or the carbon atom of the carboxylate group of E12, which are used to compute free-energy profiles, F = -ln[p(z12)], where p(z12) is the probability density. Unlike the two stable positions (inset graphic) in the wild-type (WT), which occur over a broad range of z12, the potential minima in the mutant are restricted to z12 < 0.
Intrinsic diffusion resistance of a membrane channel, mean first-passage times between its ends, and equilibrium unidirectional fluxes
Diffusion resistance is an important characteristic of channel-facilitated membrane transport and is widely used in chemical engineering, electrochemistry, and cell biophysics. It is a diffusion analog of the electrical resistance, relating the steady-state diffusive flux of solute molecules through a membrane channel with the driving force of the transport process: the solute concentration difference in the two reservoirs separated by the membrane. We derived analytical expressions for the diffusion resistance in the case of a cylindrically symmetric blocker, whose axis coincides with the axis of a cylindrical nanopore in two limiting cases, i.e., where the blocker radius changes either smoothly or abruptly (Figure 3). Comparison of our theoretical predictions with the results obtained from Brownian dynamics simulations shows good agreement between the two. We also established a general relation between the channel diffusion resistance and the mean first-passage times of the solute molecules between the channel openings. Specifically, we showed that this direction-independent characteristic of transport is equal to the sum of the direction-dependent mean first-passage times divided by the molecule partition function in the channel. Our analysis is based on consideration of the equilibrium unidirectional fluxes flowing through the channel in opposite directions. The approach is quite general in the sense that it does not apply only to any specific model of the channel and is, therefore, universally applicable to transport in channels of arbitrary shape and tortuosity, at arbitrary interaction strength of solute molecules with the channel walls. This result promises to be of great value in computing the intrinsic diffusion resistance of the channel numerically, as it allows researchers to avoid dealing with multiple problems in analyzing transport under non-equilibrium conditions.
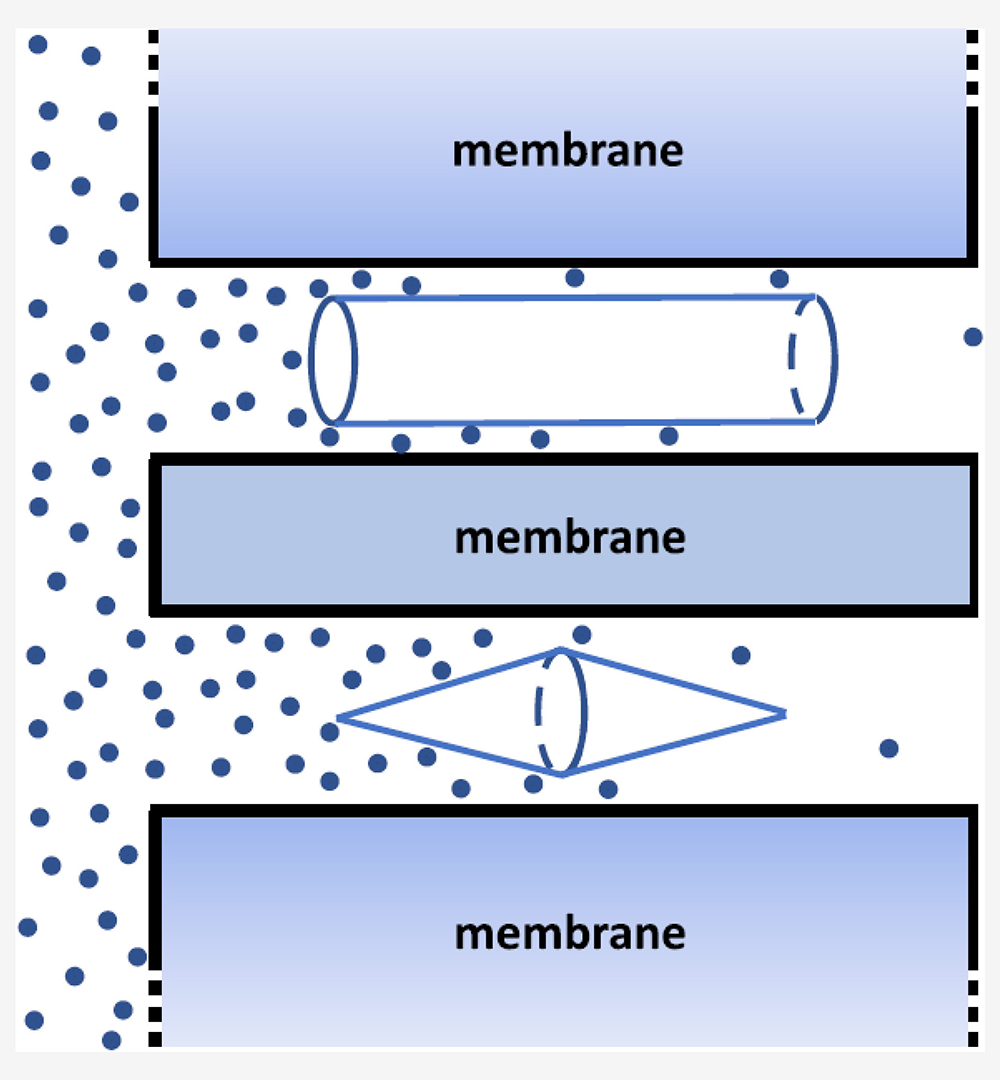
Figure 3. Blocker-induced increase in channel diffusion resistance
Schematic illustration of molecular flux through a cylindrical channel with a blocker of an abruptly varying radius (cylinder, top) and with a blocker of a smoothly varying radius (two cones connected by their bases, bottom).
Publications
- Hoogerheide DP, Rostovtseva TK, Bezrukov, SM. Voltage-activated complexation of α-synuclein with three diverse β-barrel channels: VDAC, MspA, and α-Hemolysin. Proteomics 2021 2100060.
- Rajendran M, Queralt‑Martín M, Gurnev PA, Rosencrans WM, Rovini A, Jacobs D, Abrantes K, Hoogerheide DP, Bezrukov SM, Rostovtseva TK. Restricting α‑synuclein transport into mitochondria by inhibition of α‑synuclein–VDAC complexation as a potential therapeutic target for Parkinson’s disease treatment. Cell Mol Life Sci 2022 79:368.
- Ngo VA, Queralt-Martín M, Khan F, Bergdoll L, Abramson J, Bezrukov SM, Rostovtseva TK, Hoogerheide DP, Noskov SY. The single residue K12 governs the exceptional voltage sensitivity of mitochondrial voltage-dependent anion channel gating. J Amer Chem Soc 2022 144:14564–14577.
- Berezhkovskii AM, Bezrukov SM. Intrinsic diffusion resistance of a membrane channel, mean first-passage times between its ends, and equilibrium unidirectional fluxes. J Chem Phys 2022 156:071103.
- Dagdug L, Skvortsov AT, Berezhkovskii AM, Bezrukov SM. Blocker effect on diffusion resistance of a membrane channel. Dependence on the blocker geometry. J Phys Chem B 2022 126:6016–6025.
Collaborators
- Vicente M. Aguilella, PhD, Universidad Jaume I, Castellón, Spain
- Alexandra Beilina, PhD, Cell Biology & Gene Expression Section, NIA, Bethesda, MD
- Alexander M. Berezhkovskii, PhD, Division of Computational Bioscience, CIT, NIH, Bethesda, MD
- Mark R. Cookson, PhD, Laboratory of Neurogenetics, NIA, Bethesda, MD
- Leonardo Dagdug, PhD, Universidad Autónoma Metropolitana-Iztapalapa, Mexico City, Mexico
- David Hoogerheide, PhD, National Institute of Standards and Technology, Gaithersburg, MD
- Jennifer C. Lee, PhD, Biochemistry and Biophysics Center, NHLBI, Bethesda, MD
- Ekaterina M. Nestorovich, PhD, The Catholic University of America, Washington, DC
- Sergei Y. Noskov, PhD, University of Calgary, Calgary, Canada
- Olga Protchenko, PhD, Liver Diseases Branch, NIDDK, Bethesda, MD
- Alexander Sodt, PhD, Unit on Membrane Chemical Physics, NICHD, Bethesda, MD
- Gerhard Wagner, PhD, Harvard Medical School, Cambridge, MA
- Michael Weinrich, MD, Office of the Director, NICHD, Bethesda, MD
- David L. Worcester, PhD, National Institute of Standards and Technology, Gaithersburg, MD
- Joshua Zimmerberg, MD, PhD, Section on Integrative Biophysics, NICHD, Bethesda, MD
Contact
For more information, email bezrukov@helix.nih.gov or visit https://smt.nichd.nih.gov.