Thyroid Hormone Regulation of Vertebrate Postembryonic Development

- Yun-Bo Shi, PhD, Head, Section on Molecular Morphogenesis
- Liezhen Fu, PhD, Staff Scientist
- Nga Luu, MS, Biologist
- Lingyu Bao, MS, Visiting Fellow
- Wonho Na, PhD, Visiting Fellow
- Yuta Tanizaki, PhD, Visiting Fellow
- Shouhong Wang, PhD, Visiting Fellow
- Zhaoyi Peng, BS, Graduate Student
The laboratory investigates the molecular mechanisms of thyroid hormone (TH) function during postembryonic development, a period around birth in mammals when plasma TH levels peak. The main model is the metamorphosis of Xenopus laevis and X. tropicalis, two highly related species, which offer unique but complementary advantages. The control of this developmental process by TH offers a paradigm for the study of gene function in postembryonic organ development. During metamorphosis, different organs undergo vastly different changes. Some, like the tail, undergo complete resorption, while others, such as the limb, are developed de novo. The majority of larval organs persist through metamorphosis but are dramatically remodeled to function in a frog. For example, tadpole intestine is a simple tubular structure consisting primarily of a single layer of larval epithelial cells. During metamorphosis, it is transformed into an organ with a multiply folded adult epithelium surrounded by elaborate connective tissue and muscles, through specific larval epithelial cell death and de novo development of the adult epithelial stem cells, followed by their proliferation and differentiation. The wealth of knowledge from past research and the ability to manipulate amphibian metamorphosis both in vivo by using genetic approaches or hormone treatment of whole animals, and in vitro in organ cultures offer an excellent opportunity to: (1) study the developmental function of TH receptors (TRs) and the underlying mechanisms in vivo; and (2) identify and functionally characterize genes that are critical for organogenesis, particularly the formation of the adult intestinal epithelial stem cells, during postembryonic development in vertebrates [Reference 1]. A major recent focus has been to make use of the TALEN and CRISPR/Cas9 technologies to knock out the endogenous genes for functional analyses. In addition, the recent improvements in the annotation of the Xenopus tropicalis genome allow us to carry out RNA-Seq and chromatin-immunoprecipitation (ChIP)-Seq analyses at the genome-wide level. It also allows us to adapt single-cell sequencing technology to study how TH induces cell transformations during vertebrate development. We complement our frog studies by investigating the genes found to be important for frog intestinal stem-cell development and in developing mouse intestine, by making use of the ability to carry out conditional knockout.
Thyroid hormone receptor α (TRα) controls the hindlimb metamorphosis by regulating cell proliferation and WNT signaling pathways in Xenopus tropicalis.
We have been studying Xenopus tropicalis metamorphosis as a model for postembryonic human development, and we demonstrated that TRα knockout induces precocious hindlimb development. To reveal the molecular pathways regulated by TRα during limb development, we performed chromatin immunoprecipitation and RNA sequencing on the hindlimb of premetamorphic wild-type and TRα knockout tadpoles and identified over 700 TR–bound genes up-regulated by TH treatment in wild-type but not in TRα knockout tadpoles [Reference 2]. Interestingly, most of these genes were expressed at higher levels in the hindlimb of premetamorphic TRα knockout tadpoles than in stage-matched wild-type tadpoles, suggesting their de-repression upon TRα knockout. Bioinformatic analyses revealed that the genes were highly enriched with cell-cycle– and WNT signaling–related genes. Furthermore, cell-cycle and WNT signaling pathways were also highly enriched among genes bound by TR in wild-type but not in TRα knockout hindlimb. The findings suggest that direct binding of TRα to target genes related to cell-cycle and WNT pathways is important for limb development: first by preventing precocious hindlimb formation by repressing these pathways as unliganded TR before metamorphosis; and later by promoting hindlimb development during metamorphosis by mediating TH activation of the pathways.
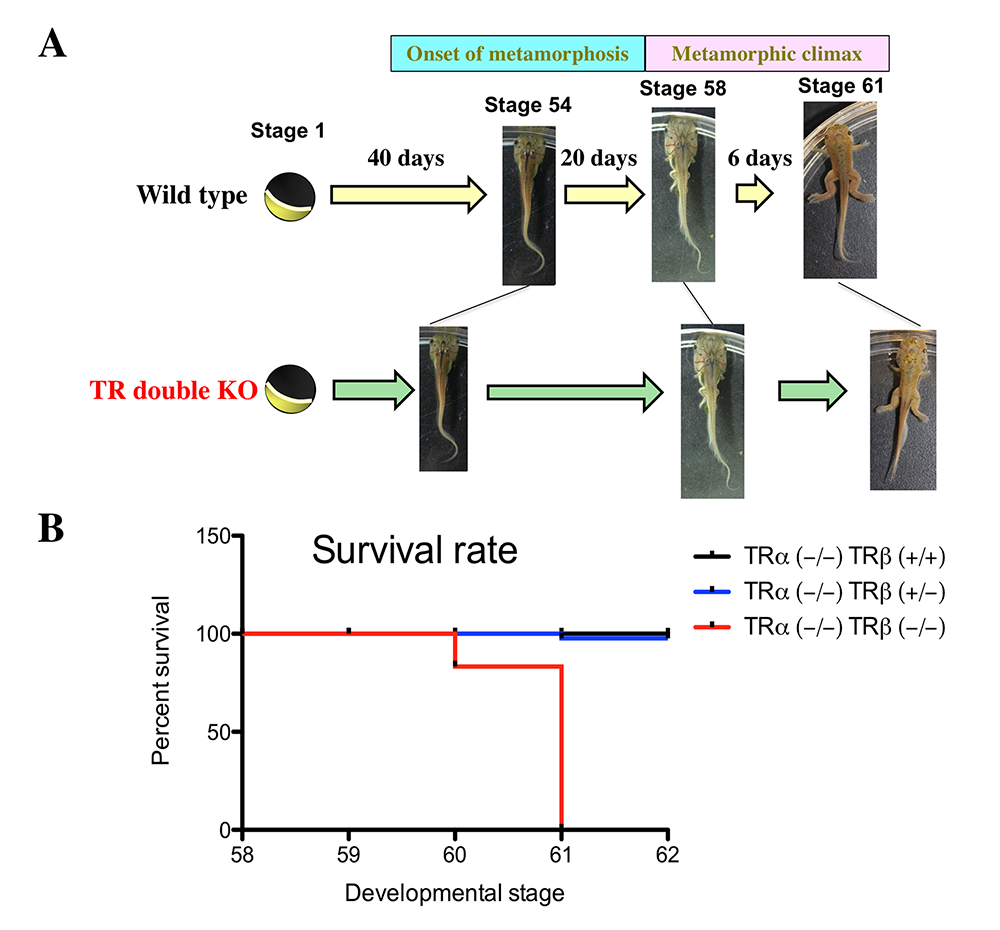
Figure 1. Effects of TR double KO on developmental rate. TR double KO leads to premature initiation of metamorphosis but slows metamorphic progress and causes lethality at the metamorphic climax.
A. TR double KO animals take a shorter time to reach the onset of metamorphosis (stage 54), indicating accelerated premetamorphic development. Once metamorphosis begins, the KO animals take longer to reach the beginning of metamorphic climax (stage 58) and also develop more slowly during the climax stages, between stages 58 and 61. The length of each stage indicates the relative time needed for development between two adjacent stages.
B. Tadpoles without any TR die during the climax of metamorphosis. The tadpoles of mixed genotypes at stage 58 were allowed to develop to stage 62 and were genotyped at stage 62 or when they died during this developmental period. The survival rate for each of the three genotypes, trα–/–trβ+/+, trα–/–trβ+/–, and trα–/–trβ–/–, was thus obtained and plotted. Note that no double knockout tadpoles developed to stage 62 and that a single copy of trβ+/– was sufficient for the animal to complete metamorphosis and develop into a reproductive adult.
Transcriptome profiling reveals gene regulation programs underlying tail development in the Ornamented Pygmy frog Microhyla fissipes.
For comparative studies on the role of TH in anuran metamorphosis, we had previously carried out RNA-Seq analysis of the TH–induced gene expression program during tail resorption in Microhyla fissipes tadpoles. Parallel to the metamorphic study, we also analyzed the tail at different developmental stages. Tadpole tail develops from the tailbud, an apparently homogenous mass of cells at the posterior of the embryo. While much progress has been made in understanding the origin and the induction of the tailbud, its subsequent outgrowth and differentiation have received much less attention, particularly with regard to global gene expression changes. By using RNA-Seq with single-molecule real time (SMRT) sequencing and further analyses, we revealed the transcriptome profiles at four key stages of tail development, from a small tailbud to the onset of feeding (S18, S19, S21, and S28) in Microhyla fissipes [Reference 3]. We obtained 48,826 transcripts and discovered 8,807 differentially expressed transcripts (DETs, q < 0.05) among these four developmental stages. We functionally classified the DETs by using GO (gene ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) analyses and revealed 110 significantly enriched GO categories and six highly enriched KEGG pathways (protein digestion and absorption; ECM-receptor interaction; pyruvate metabolism; fatty acid degradation; valine, leucine, and isoleucine degradation; and glyoxylate and dicarboxylate metabolism) that are likely critically involved in developmental changes in the tail. In addition, analyses of DETs between any two individual stages demonstrated the involvement of distinct biological pathways/GO terms at different stages of tail development. Furthermore, the most dramatic changes in gene expression profile are those between S28 and any of the other three stages. The upregulated DETs at S28 are highly enriched in “myosin complex” and “potassium channel activity,” which are important for muscle contraction, a critical function of the tail that the animal needs by the end of embryogenesis. Additionally, many DETs and enriched pathways during tail development, which we discovered, such as the HDAC1, Hes1, and Hippo signaling pathway, have also been reported to be vital for the tissue/organ regeneration, suggesting conserved functions between development and regeneration.
The sperm-associated antigen 7 gene (spag7) is activated by TH during Xenopus tropicalis metamorphosis via a thyroid hormone–response element within the first intron.
We previously identified spag7 as a candidate TH target gene that is potentially involved in adult stem cell development and/or proliferation during intestinal metamorphosis. To investigate whether TH regulates spag7 directly at the transcriptional level via TR, we first conducted qRT-PCR to analyze its expression during natural and TH–induced metamorphosis and found that spag7 was up-regulated during natural metamorphosis in the intestine, tail, brain, and hindlimb, peaking at the climax of metamorphosis in all those organs, and upon TH treatment of premetamorphic tadpoles. Next, we demonstrated that an intronic thyroid hormone–response element (TRE) in spag7, first identified through bioinformatic analysis, could bind to TR in vitro and in vivo during metamorphosis. A dual luciferase assay utilizing a reconstituted frog oocyte transcription system showed that the TRE could mediate promoter activation by liganded TR. The results indicate that spag7 expression is directly regulated by TH through the TRE in the first intron during metamorphosis, implicating a role for spag7 early during TH–regulated tissue remodeling and resorption [Reference 4].
Figure 2. Intestinal metamorphosis involves the formation of clusters of proliferating, undifferentiated epithelial cells at the climax.
Tadpoles at premetamorphic stage 54 (A), climax, stage 62 (B), and the end of metamorphosis, stage 66 (C) were injected with 5-ethynyl-2′-deoxyuridine (EdU) one hour before sacrifice. Cross-sections of the intestine from the resulting tadpoles were double-stained by EdU labeling of newly synthesized DNA and by immunohistochemistry of IFABP (intestinal fatty acid–binding protein), a marker for differentiated epithelial cells. The dotted lines depict the epithelium-mesenchyme boundary. Note that there are few EdU–labeled proliferating cells in the epithelium and that they express IFABP at premetamorphosis (A) and increase in the form of clustered cells (proliferating adult stem cells), which lack IFABP at the climax of metamorphosis (B). At the end of metamorphosis, EdU–labeled proliferating cells are localized mainly in the troughs of the epithelial folds, where IFABP expression is low (C). ep, epithelium; ct, connective tissue; m, muscles; l, lumen.
Thyroid hormone receptor α controls larval intestinal epithelial cell death by regulating the CDK1 pathway.
We have been studying intestinal remodeling during Xenopus tropicalis metamorphosis as a model in which to study TR function in adult organ development. By using ChIP (chromatin immunoprecipitation)-Seq, we identified over 3,000 TR–bound genes in the intestine of premetamorphic wild type or TRα (the major TR expressed during premetamorphosis)–knockout tadpoles [Reference 5]. Surprisingly, cell cycle–related GO (gene ontology) terms and biological pathways were highly enriched among TR target genes, even though the first major event during intestinal metamorphosis is larval epithelial cell death, and TRα knockout drastically reduced this enrichment. More importantly, treatment of tadpoles with cell-cycle inhibitors blocked TH–induced intestinal remodeling, especially larval epithelial cell death, suggesting that TRα–dependent cell cycle activation is important for TH–induced apoptosis during intestinal remodeling.
Additional Funding
- Japan Society for the Promotion of Science (JSPS) fellowship for Yuta Tanizaki
- FY22 NICHD Early Career Award for Yuta Tanizaki
Publications
- Shi Y-B, Shibata Y, Tanizaki Y, Fu L. The development of adult intestinal stem cells: Insights from studies on thyroid hormone-dependent anuran metamorphosis. Vitam Horm 2021 116:269–293.
- Tanizaki Y, Shibata Y, Zhang H, Shi Y-B. Thyroid hormone receptor α controls the hindlimb metamorphosis by regulating cell proliferation and Wnt signaling pathways in Xenopus tropicalis. Int J Mol Sci 2022 23(3):1223.
- Wang S, Liu L, Shi Y-B, Jiang J. Transcriptome profiling reveals gene regulation programs underlying tail development in the Ornamented Pygmy frog Microhyla fissipes. Cell Biosci 2021 26:1001–12.
- Fu L, Crawford L, Tong A, Luu N, Tanizaki Y, Shi Y-B. Sperm associated antigen 7 (SPAG7) is activated by T3 during Xenopus tropicalis metamorphosis via a thyroid hormone response element (TRE) within the first intron. Dev Growth Differ 2022 64:48–58.
- Tanizaki Y, Zhang H, Shibata Y, Shi Y-B. Thyroid hormone receptor α controls larval intestinal epithelial cell death by regulating the CDK1 pathway. Commun Biol 2022 5:112.
Collaborators
- Steven Coon, PhD, Molecular Genomics Core, NICHD, Bethesda, MD
- Eiichi Hinoi, PhD, Kanazawa University Graduate School, Kanazawa, Japan
- James Iben, PhD, Molecular Genomics Core, NICHD, Bethesda, MD
- Jianping Jiang, PhD, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, China
- Tianwei Li, PhD, Molecular Genomics Core, NICHD, Bethesda, MD
- Susan Mackem, MD, PhD, Cancer and Developmental Biology Laboratory, Center for Cancer Research, NCI, Frederick, MD
- Bingyin Shi, MD, Xi’an Jiaotong University School of Medicine, Xi'an, China
- Hari Shroff, PhD, Section on High Resolution Optical Imaging, NIBIB, Bethesda, MD
- Guihong Sun, PhD, Wuhan University School of Medicine, Wuhan, China
- Peter Taylor, PhD, University of Dundee, Dundee, United Kingdom
- Henry Zhang, PhD, Bioinformatics and Scientific Programming Core, NICHD, Bethesda, MD
Contact
For more information, email shi@helix.nih.gov or visit https://smm.nichd.nih.gov.