Molecular Nature and Functional Role of Dendritic Voltage-Gated Ion Channels

- Dax Hoffman, PhD, Head, Molecular Neurophysiology and Biophysics Section
- Jiahua Hu, PhD, Staff Scientist
- Lin Lin, PhD, Microbiologist
- Ying Liu, MD, Biologist
- Cole Malloy, PhD, Postdoctoral Fellow
- Jon Murphy, PhD, Postdoctoral Fellow
- Meghyn Welch, PhD, Postdoctoral Fellow
- Maisie Ahern, BS, Postbaccalaureate Fellow
- Ashley Pratt, BS, Postbaccalaureate Fellow
The central nervous system (CNS) underlies all our experiences, actions, emotions, knowledge, and memories. With billions of neurons each firing hundreds of times per second, the complexity of the brain is stunning. To pare down the task of understanding something so complex, our research approach calls for studying the workings of a single central neuron: the pyramidal neuron from the CA1 region of the hippocampus. In humans, the hippocampus is essential for long-term memory and is among the first brain regions affected by epilepsy and Alzheimer’s disease. To understand how the hippocampus stores and processes information, we focus on the CA1 pyramidal neuron, one of its principal cell types. Each of these cells receives tens of thousands of inputs onto its dendrites, and it is commonly thought that information is stored by altering the strength of individual synapses (synaptic plasticity). Recent evidence suggests that the regulation of synaptic surface expression of glutamate receptors can, in part, determine synaptic strength. However, the dendrites contain an abundance of ion channels that are involved in receiving, transforming, and relaying information in the dendrites, adding an additional layer of complexity to neuronal information processing.
We found that the A-type potassium channel subunit Kv4.2 is highly expressed in the dendritic regions of CA1 neurons in the hippocampus and, as one of the primary regulators of dendritic excitability, plays a pivotal role in information processing. Kv4.2 is targeted for modulation during the types of plasticity thought to underlie learning and memory. Moreover, we found that the functional expression level of Kv4.2 regulates the subtype expression of NMDA–type glutamate receptors, the predominant molecular devices controlling synaptic plasticity and memory. We are currently following up on these findings with more detailed investigations into the mechanisms of activity-dependent Kv4.2 regulation. In addition, we have begun to investigate the role of dendritic voltage-gated potassium and calcium channels in neuronal development and developmental disorders.
Role of voltage-gated ion channels in synaptic development and disease
Isomerase regulation of potassium channel trafficking and function.
We recently identified a novel molecular cascade initiated by the activation of p38 kinase and subsequent isomase Pin1–dependent isomerization of a C-terminal motif (T607) in Kv4.2 that triggers dissociation from its auxiliary subunit DPP6, a reduction in the voltage-gated K+ channel IA, and an increase in neuronal excitability. Pin1 is a prolyl isomerase that selectively binds to and isomerizes phospho-Ser/Thr-Pro (pSer/Thr-Pro) bonds. Mis-regulation of Pin1 plays an important role in a growing number of pathological conditions, including Alzheimer's disease, where it may protect against age-dependent neurodegeneration. Using biochemical and electrophysiological techniques, we showed that Pin1 activity is required for the dissociation of the Kv4.2DPP6 complex and that this action alters neuronal excitability. To investigate the consequences of this cascade on behavior and neuronal physiology, we used CRISPR-Cas9 techniques to generate a knockin mouse in which the isomerase binding site is specifically abolished (Kv4.2TA). The mice are viable and appear normal, although the activity-dependent dissociation of the Kv4.2–DPP6 complex is impaired. Kv4.2TA mice exhibit normal initial learning and memory in the Morris Water Maze; however, they exhibited better ‘reversal’ learning in Morris Water Maze than did wild-type (WT) mice. The data strongly support the idea that activity-dependent regulation of Kv4.2 plays an important role in cognitive flexibility. Cognitive flexibility is the ability to appropriately adjust one’s behavior to a changing environment and is impaired in various neurodevelopmental disorders, such as the autism spectrum disorder.
Considering the finding that Kv4.2TA mice exhibit enhanced cognitive flexibility, ongoing experiments are investigating potential differences in synaptic properties between WT and Kv4.2TA mice. To determine the cellular/molecular correlate of this cognitive phenotype, Cole Malloy used patch clamp electrophysiology in hippocampal CA1 pyramidal cells from Kv4.2TA and WT mice to record several forms of synaptic plasticity. Kv4.2TA mice exhibit basal synaptic transmission that is similar to that of WT mice, as evidenced by comparable mini excitatory synaptic currents (mEPSCs), synaptic release probability (paired-pulse ratios), and evoked synaptic transmission magnitude. Additionally, long-term plasticity from a basal state is preserved relative to WT in stratum radiatum of the hippocampus. We performed single-cell measures of spike timing–dependent long-term potentiation (STD-LTP) and long-term depression (LTD) in CA1 pyramidal neurons in acute hippocampal slices. We observed no change in either STD-LTP, NMDA–dependent LTD, or mGluR–dependent LTD in Kv4.2TA mice compared with WT when recorded from a basal state. However, intriguingly, there was a significant enhancement in the reversal of STD-LTP (depotentiation) magnitude in Kv4.2TA mice, which appears to be driven by differences in NMDA–mediated transmission. This suggests a synapse state–dependent difference in synaptic plasticity in CA1 stratum-radiatum of the hippocampus facilitated by loss of dynamic regulation of the Kv4.2 complex. We have thus revealed a novel metaplasticity mechanism in a Kv4.2 mouse model. Further investigations into the correlation of this plasticity and the cognitive flexibility phenotype exhibited by this mouse model, as well as into more detailed molecular mechanisms, are on-going.
Seizure or neuronal activity leads to Kv4.2 protein degradation. We found that Kv4.2 degradation is dependent on the above Pin1 mechanism, given that Kv4.2 trafficking and degradation are abolished in Kv4.2TA mice. Seizure or neuronal activity triggers Kv4.2 phosphorylation and subsequent isomerization by Pin1, which results in Kv4.2 dissociation with the peptidase Dpp6 and internalization. Internalized Kv4.2 underwent ubiquitination and degradation. In order to examine whether DPP6 is required for this process, we employed DPP6 knock-out (KO) mice to test kainic acid–induced Kv4.2 protein loss. We found that seizure-induced Kv4.2 degradation is abolished in DPP6 KO mice, suggesting that seizure-induced Kv4.2 protein loss occurred in DPP6–containing Kv4.2 complex. Interestingly, we found that seizure-induced Kv4.2 phosphorylation at the Pin1 isomerization site is also abolished in DPP6 KO mice, while induction of p38 activity is normal in DPP6 KO, suggesting that seizure-induced Kv4.2 phosphorylation occurred in DPP6–containing Kv4.2 complex. These data are consistent with and support the notion that seizure of neuronal activity induces phosphorylation of DPP6–containing Kv4.2 complex, which results in Dpp6-Kv4.2 dissociation and internalization and subsequent ubiquitination and degradation.
Ca2+ regulation of potassium channel function
Jonathan Murphy found that Ca2+ entry mediated by the voltage-gated Ca2+ channel subunit Cav2.3 regulates Kv4.2 function both in a heterologous expression system and endogenously in CA1 pyramidal neurons through Ca2+-binding auxiliary subunits known as K+ channel–interacting proteins (KChIPs). KChIPs are calcium-sensing molecules containing four EF-hands, which are dysregulated in several diseases and disorders, including epilepsy, Huntington's disease, and Alzheimer's disease. We identified Cav2.3 as a Kv4.2–interacting protein in a proteomic screen and confirmed Cav2.3-Kv4.2 complex association using several techniques. Murphy characterized a KChIP–independent interaction between Cav2.3 and Kv4.2 using immunofluorescence colocalization, coimmunoprecipitation, electron microscopy, FRAP, and FRET. We found that Ca2+ entry via Cav2.3 increases Kv4.2–mediated whole-cell current in part as a result of an increase in Kv4.2 surface expression. In hippocampal neurons, pharmacological block of Cav2.3 reduced whole-cell IA. We also found a reduction in whole-cell IA in Cav2.3 KO mice mouse neurons, with a loss of the characteristic dendritic IA gradient. Furthermore, the loss of Cav2.3 function leads to the enhancement of AMPA receptor–mediated synaptic currents and NMDA receptor–mediated spine Ca2+ influx. The findings reveal that an intermolecular Cav2.3–Kv4.2 complex impacts synaptic integration in CA1 hippocampal neurons.
DPP6 impacts brain development, function and Alzheimer’s disease/dementia
In 2020 Lin Lin reported [Reference 6] that the novel structures in hippocampal area CA1 are significantly more prevalent in DPP6-KO aging mice than in WT mice and are also observed earlier during development in DPP6-KO mice. These novel structures apparently derived from degenerating presynaptic terminals, as clusters of large puncta that colocalize NeuN, synaptophysin, and chromogranin A, and also partially label for MAP2, β-amyloid, β-amyloid precursor protein (APP), α-synuclein, and phosphorylated tau, with synapsin-1 and VGluT1 labeling on their periphery.
Following these results, Lin Lin recently found that DPP6-KO mice show enhanced neurodegeneration associated with Alzheimer pathology. By using immunofluorescence and electron microscopy, we confirmed that both APP and β-amyloid are prevalent in these novel structures; and we showed, with immunofluorescence, that similar novel structures are found in the human hippocampal CA1 of Alzheimer’s disease donors. In aged mice, we used in vivo MRI to show reduced size in DPP6-KO brain and hippocampus. Aging DPP6-KO hippocampi contained fewer total neurons and greater neuron death and showed diagnostic biomarkers of Alzheimer’s disease, including accumulation of β-amyloid and APP and increase in expression of hyper-phosphorylated tau. The β-amyloid and phosphorylated tau pathologies were associated with neuro-inflammation characterized by increases in microglia and astrocytes. Levels of pro-inflammatory or anti-inflammatory cytokines increased in aging DPP6-KO mice. We also showed that aging DPP6-KO mice display circadian dysfunction, a common symptom of Alzheimer’s disease. Together these results indicate that aging DPP6-KO mice show symptoms of enhanced neurodegeneration reminiscent of dementia associated with a novel structure resulting from synapse loss and neuronal death.
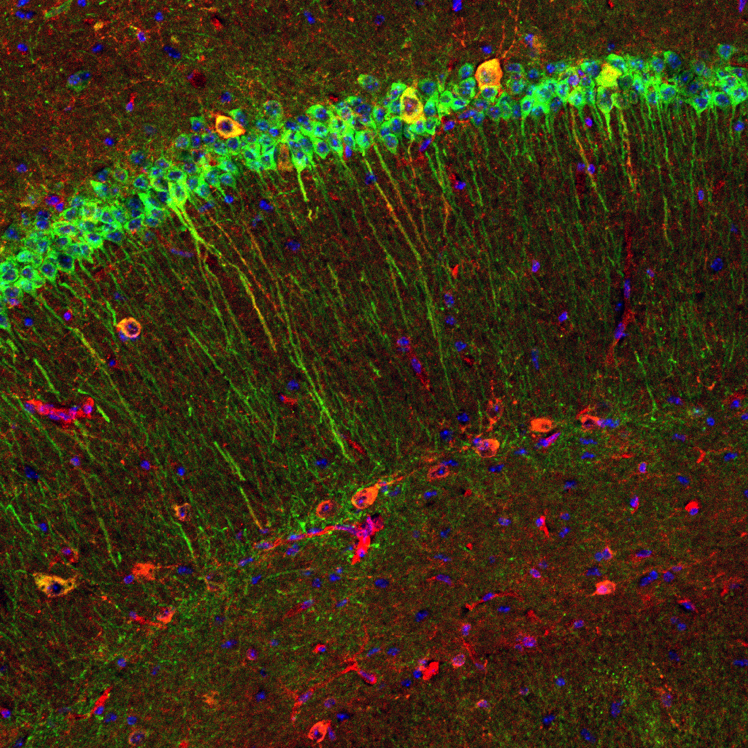
Figure 1. Amyloid accumulation in the CA1 hippocampus of aging DPP6-KO mice
Immunofluorescence image from an 8-week-old DPP6-KO mouse brain shows diffuse labeling for β-amyloid (red) in some pyramidal neuron cell bodies and apical dendrites (labeled in green for MAP2). In addition, labeling for β-amyloid is concentrated in some interneurons (large, yellow).
Publications
- Murphy JG, Gutzmann JJ, Lin L, Hu J, Petralia RS, Wang YX, Hoffman DA. R-type voltage-gated Ca2+ channels mediate A-type K+ current regulation of synaptic input in hippocampal dendrites. Cell Rep 2022 38(3):110264.
- Lin L, Petralia RS, Holtzclaw L, Wang YX, Abebe D, Hoffman DA. Alzheimer's disease/dementia-associated brain pathology in aging DPP6-KO mice. Neurobiol Dis 2022 174:105887.
- Malloy C, Ahern M, Lin L, Hoffman DA. Neuronal roles of the multifunctional protein dipeptidyl peptidase-like 6 (DPP6). Int J Mol Sci 2022 23(16):9184.
- Hu JH, Malloy C, Tabor GT, Gutzmann JJ, Liu Y, Abebe D, Karlsson RM, Durell S, Cameron HA, Hoffman DA. Activity-dependent isomerization of Kv4.2 by Pin1 regulates cognitive flexibility. Nat Commun 2020 11(1):1567.
- Hu JH, Malloy C, Hoffman DA. P38 regulates kainic acid-induced seizure and neuronal firing via Kv4.2 phosphorylation. Int J Mol Sci 2020 21(16):5921.
- Lin L, Petralia RS, Lake R, Wang YX, Hoffman DA. A novel structure associated with aging is augmented in the DPP6-KO mouse brain. Acta Neuropathol Commun 2020 8(1):197.
Collaborators
- Heather Cameron, Section on Neuroplasticity, NIMH, Bethesda, MD
- Constantine A. Stratakis, MD, D(med)Sci, Section on Endocrinology and Genetics, NICHD, Bethesda, MD
Contact
For more information, email hoffmand@mail.nih.gov or visit https://hoffmanlab.nichd.nih.gov.