Regulation of Childhood Growth

- Jeffrey Baron, MD, Head, Section on Growth and Development
- Kevin Barnes, PhD, Senior Research Assistant
- Julian Lui, PhD, Staff Scientist
- Youn Hee Jee, MD, Staff Clinician
- Bijal Kikani, BS, Postbaccalaureate Fellow
- Adrian Temnycky, BS, Postbaccalaureate Fellow
Children grow taller because their bones grow longer. The bone elongation occurs at the growth plate, a thin layer of cartilage found near the ends of juvenile bones. In the growth plates, new cartilage is produced through chondrocyte proliferation, hypertrophy, and cartilage matrix synthesis, and then the newly formed cartilage is remodeled into bone. The process, termed endochondral ossification, results in bone elongation, which causes children to grow in height (linear growth). Consequently, mutations in genes that regulate growth plate chondrogenesis cause abnormal bone growth and short stature in children. Depending on the severity and nature of the genetic abnormality, the phenotype can range from chondrodysplasias with short, malformed bones, to severe, often disproportionate, short stature, to mild proportionate short stature. If the genetic defect affects tissues other than the growth plate cartilage, the child may present with a more complex syndrome that includes further clinical abnormalities.
We investigate the cellular and molecular mechanisms governing childhood growth and development. We focus particularly on growth at the growth plate, which drives bone elongation and therefore determines height. One goal of this work is to gain insight into the many human genetic disorders that cause childhood growth failure or overgrowth. A second goal is to develop new treatments for children with severe growth disorders.
Novel genetic causes of childhood growth disorders
For many children who are brought to medical attention for linear growth disorders, clinical, laboratory, and genetic evaluation fails to identify the underlying etiology. Genome-wide association studies and molecular studies of growth-plate biology suggest that there are hundreds of genes that control linear growth. Therefore, there are likely many genetic causes of linear growth disorders that remain to be discovered.
To discover new genetic causes of childhood growth disorders, we invite families with monogenic growth disorders to the NIH Clinical Center, where we evaluate the clinical, biochemical, and radiological features of the condition. We then obtain DNA samples from informative family members and use powerful genetic approaches, including SNP arrays, to detect deletions, duplications, mosaicism, and uniparental disomy, combined with exome sequencing to detect single-nucleotide variants and small insertions/deletions in coding regions and splice sites. When sequence variants that are likely to cause the disorder are identified, we study in the laboratory the variants and the genes in which they occur to confirm that the variant is pathogenic, to elucidate the pathogenesis of the disorder, and to explore the role of the gene in normal growth.
Recently, such analyses showed that mutations in a gene called QRICH1 impairs growth at the growth plates, causing short stature [Reference 1]. We studied a child with short stature, irregular growth plates of the proximal phalanges, developmental delay, and mildly dysmorphic facial features. Exome sequencing identified a de novo, heterozygous, nonsense mutation in QRICH1. Our in vitro studies confirmed that the mutation impaired expression of the QRICH1 protein. Knockdown of Qrich1 in primary mouse epiphyseal chondrocytes mediated by siRNA caused downregulation of gene expression associated with hypertrophic differentiation, a step that is critical for bone elongation. We then identified an unrelated individual with another heterozygous de novo nonsense mutation in QRICH1, who had a similar phenotype. A recently published study identified QRICH1 mutations in three patients with developmental delay, one of whom had short stature. Our findings indicate that QRICH1 mutations cause not only developmental delay but also a chondrodysplasia characterized by diminished linear growth and abnormal growth plate morphology resulting from impaired growth plate chondrocyte hypertrophic differentiation [Reference 1].
Molecular and cellular mechanisms by which specific genes and pathways regulate childhood growth
Our group also studies the fundamental mechanisms governing skeletal growth. Recently, we focused on why bones at different anatomical locations vary dramatically in size [Reference 2]. For example, human femurs are 20-fold longer than the phalanges in the fingers and toes. The mechanisms responsible for such size differences are poorly understood. Bone elongation occurs at the growth plates and advances rapidly in early life but then progressively slows as a consequence of a developmental program termed growth plate senescence. This developmental program includes declines in cell proliferation and hypertrophy, depletion of cells in all growth plate zones, and extensive underlying changes in the expression of growth-regulating genes. We found evidence that these functional, structural, and molecular senescent changes occur earlier in the growth plates of smaller bones (metacarpals, phalanges) than in the growth plates of larger bones (femurs, tibias), and that such differential aging contributes to the disparities in bone length (Figure 1). We also found evidence that the molecular mechanisms that underlie the differential aging between different bones involve modulation of critical paracrine regulatory pathways, including Igf, Bmp, and Wnt signaling. Taken together, the findings reveal that the striking disparities in lengths of different bones, which characterize normal mammalian skeletal proportions, are achieved in part by modulating the progression of growth-plate senescence [Reference 2].
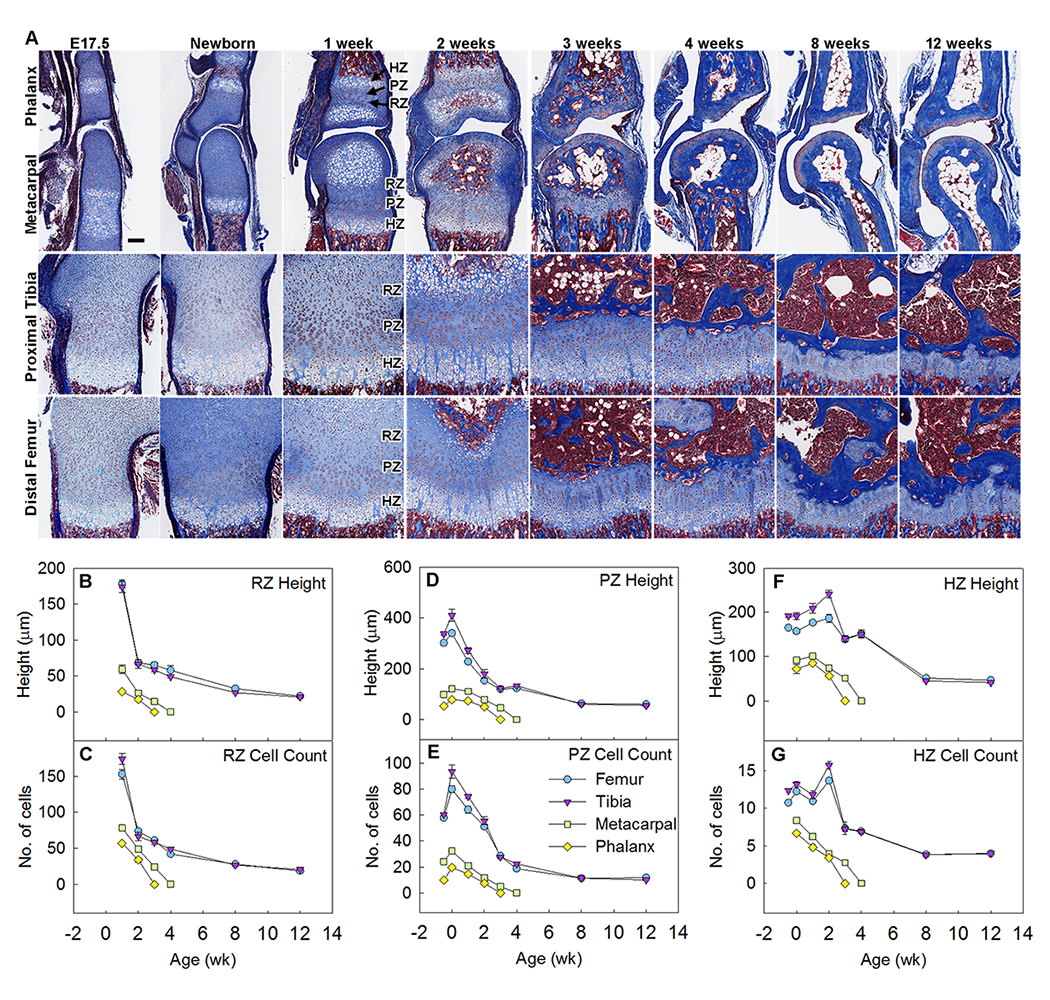
Click image to view.
Figure 1. Growth plate senescence is more advanced in shorter bones than in longer bones.
Masson Trichrome–stained histological sections of proximal tibias, distal femurs, distal metacarpals, and proximal forelimb phalanges from C57BL/6 mice at various ages. Cartilage matrix stains light blue, bone matrix dark blue. Epiphyseal fusion (disappearance of growth plate) occurs at approximately 3 weeks in phalanges and 4 weeks in metacarpals but has not yet occurred at 12 weeks in tibias or femurs. Scale bar, 100 μm. (B–G) Quantitative histological measurements of RZ height (panel B) and cell count (panel C), PZ height (panel D) and cell count per column (panel E), HZ height (panel F) and cell count per column (panel G), in each of the 4 growth plates at various ages. HZ: hypertrophic zone; PZ: proliferative zone; RZ: resting zone.
We also explored the transdifferentiation of growth plate chondrocytes into osteoblasts [Reference 3]. In the postnatal growth plate, as hypertrophic chondrocytes approach the chondro-osseous junction, they may undergo apoptosis or directly transdifferentiate into osteoblasts. The molecular mechanisms governing the switch in cell lineage are poorly understood. We found that, in hypertrophic chondrocytes, the physiological downregulation of Sox9 (a transcription factor critical for chondrogenesis) is associated with upregulation of osteoblast-associated genes (such as Mmp13, Col1a1, Ibsp), before the chondrocytes enter the metaphyseal bone. In transgenic mice that continued to express Sox9 in all cells derived from the chondrocytic lineage, upregulation of these osteoblast-associated genes in the hypertrophic zone failed to occur. Furthermore, lineage tracing experiments showed that, in transgenic mice expressing Sox9, the number of chondrocytes transdifferentiating into osteoblasts was markedly reduced (Figure 2). Collectively, our findings suggest that Sox9 downregulation in hypertrophic chondrocytes promotes expression of osteoblast-associated genes in hypertrophic chondrocytes and promotes the subsequent transdifferentiation of these cells into osteoblasts [Reference 3].
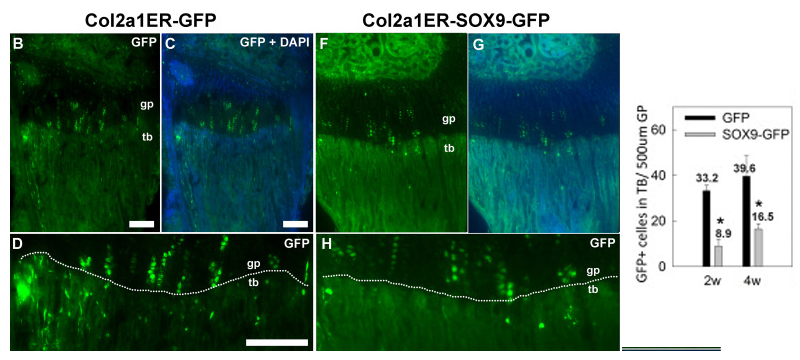
Click image to view.
Figure 2. Transgenic Sox9 expression suppressed transition of chondrocytes into trabecular bone.
Lineage-tracing experiments were performed by crossing mice with tamoxifen-inducible cre expression driven by a collagen 2 promoter (Col2a1ER-cre) with either the transgenic Sox9-GFP mice or transgenic mice expressing only GFP. Fluorescent microscopy (B–G lower magnification, D–H higher magnification) showed that GFP–positive chondrocyte columns were present in the growth plate (gp, area above the white dotted line) in both Col2a1ER-GFP and Col2a1ER-Sox9-GFP mice. However, in mice with Sox9 misexpression, there were significantly fewer GFP–positive cells in the metaphyseal trabecular bone (tb, area below the white dotted line) at both 2 and 4 weeks of age (graph). *, P<0.05, ANOVA.
New treatment approaches for growth plate disorders
Currently, treatment approaches for linear growth disorders are limited. Recombinant human growth hormone (GH) is used for both GH deficiency and certain causes of short stature not attributable to GH deficiency. However, the efficacy of GH treatment is often suboptimal in severe, non–GH deficient conditions such as skeletal dysplasias. Consequently, the conditions for which treatment is most needed, such as achondroplasia, are often the conditions for which GH is least effective. Because GH has limited efficacy for severe disease and significant known and potential adverse effects, better treatments for growth disorders are needed.
In addition to GH, there are also many paracrine factors that positively regulate growth-plate chondrogenesis and might therefore be used therapeutically. The development of these paracrine factors into effective treatment has been hampered by their mechanism of action; the growth factors are produced locally and act locally in the growth plate and thus do not lend themselves to systemic therapeutic approaches. We envision that these locally acting molecules could be targeted to the growth plate by linking them to cartilage-binding proteins, such as antibody fragments. When administered systemically, such hybrid molecules would be preferentially taken up by growth plate cartilage and thus might greatly augment the therapeutic effect on the target organ while diminishing adverse effects due to action on other tissues. Similarly, growth-promoting endocrine factors, such as GH and IGF-I, might be targeted to the growth plate to enhance the therapeutic effects on chondrogenesis and reduce adverse effects on nontarget tissues.
For this purpose, we developed cartilage-targeting single-chain human antibody fragments and then created fusion proteins of such antibody fragments, combined with insulin-like growth factor 1 (IGF-1), an endocrine/paracrine factor that positively regulates chondrogenesis [Reference 4]. The fusion proteins retained both cartilage-binding and IGF-1 biological activity and were able to stimulate bone growth in an organ culture system. Using a growth hormone–deficient mouse model, we found evidence that subcutaneous injections of the fusion proteins had greater on-target efficacy at the growth plate (Figure 3) and less off-target effect than IGF-1 alone. Our findings provide proof-of-principle that targeting therapeutics to growth plate cartilage has the potential to improve treatment for childhood growth disorders [Reference 4].
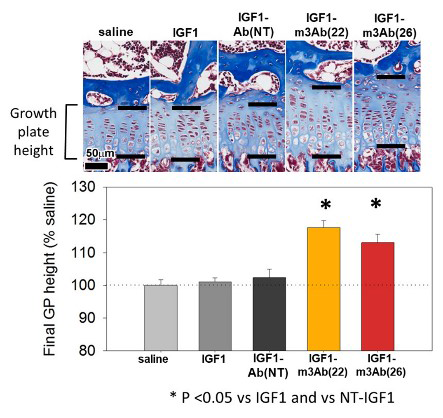
Click image to view.
Figure 3. Effect of IGF-1 on growth plate height in lit (GH–deficient) mice
Treatment with fusion proteins that combine IGF-1 with cartilage-targeting fragments [IGF1-m3Ab(22), IGF1-m3Ab(26)] increased growth plate height, whereas neither IGF-1 itself nor a nontargeted fusion protein [IGF1-Ab(NT)] had an effect. *p < 0.05.
Additional Funding
- NIH U01 award: 1U01HD086838-01A1 (2017-2021, ongoing): “Genetic Diagnosis of Childhood Growth Disorders”
Publications
- Lui JC, Jee YH, Lee A, Yue S, Wagner J, Donnelly D, Vogt K, Baron J. QRICH1 mutations cause a chondrodysplasia with developmental delay. Clin Genet 2019;95:160-164.
- Lui JC, Jee YH, Garrison P, Iben JR, Yue S, Ad M, Nguyen Q, Kikani B, Wakabayashi Y, Baron J. Differential aging of growth plate cartilage underlies differences in bone length and thus helps determine skeletal proportions. PLoS Biol 2018;23:16(7):e2005263.
- Lui JC, Yue S, Lee A, Kikani B, Temnycky A, Barnes KM, Baron J. Persistent Sox9 expression in hypertrophic chondrocytes suppresses transdifferentiation into osteoblasts. Bone 2019;125:169-177.
- Lui JC, Colbert M, Cheung CS, Ad M, Lee A, Zhu Z, Barnes K, Dimitrov DS, Baron J. Cartilage-targeted IGF-1 treatment to promote longitudinal bone growth. Mol Ther 2019;27:673-680.
- Jee YH, Baron J, Nilsson O. New developments in the genetic diagnosis of short stature. Curr Opin Pediatr 2018;30:541-547.
Collaborators
- Greti Aguilera, MD, Scientist Emeritus, Section on Endocrine Physiology, NICHD, Bethesda, MD
- Angela Delaney Freedman, MD, Office of the Clinical Director, NICHD, Bethesda, MD
- Lijin Dong, PhD, Genetic Engineering Core, NEI, Bethesda, MD
- Ellen Leschek, MD, Division of Diabetes, Endocrinology, and Metabolic Diseases, NIDDK, Bethesda, MD
- Thomas Markello, MD, PhD, Undiagnosed Diseases Program, NHGRI, Bethesda, MD
- Ola Nilsson, MD, PhD, Karolinska Institute, Stockholm, Sweden
- Sally Radovick, MD, Rutgers Biomedical and Health Sciences, Robert Wood Johnson Medical School, New Brunswick, NJ
- Katherine W. Roche, PhD, Receptor Biology Section, NINDS, Bethesda, MD
- Jack Yanovski, MD, PhD, Section on Growth and Obesity, NICHD, Bethesda, MD
Contact
For more information, email jeffrey.baron@nih.gov or visit http://baron.nichd.nih.gov.


