Translational Biophotonics in Developmental Disorders and Diseases

- Amir H. Gandjbakhche, PhD, Head, Section on Translational Biophotonics
- Afrouz Anderson, PhD, Postdoctoral Fellow
- Emma Condy, PhD, Postdoctoral Fellow
- Helga De Oliveira Ramirez, PhD, Postdoctoral Fellow
- Kosar Khaksari, PhD, Postdoctoral Fellow
- Siddharth Khare, PhD, Postdoctoral Fellow
- Thien Nguyen, PhD, Postdoctoral Fellow
- Hadis Dashtestani, MS, Intramural Research Training Award Student
- Aisling Casey, BS, Postbaccalaureate Fellow
- Hye Soo Chun, BS, Postbaccalaureate Fellow
- Joy Cui, BS, Postbaccalaureate Fellow
- John Millerhagen, BS, Postbaccalaureate Fellow
- Douglas Harrison, BS, Postbaccalaureate Fellow
- Siamak Aram, PhD, Guest Researcher
- Yasaman Ardeshirpour, PhD, Guest Researcher
- Marc Bornstein, PhD, Special Volunteer
- Han-Shin Hahn, PhD, Special Volunteer
Brain imaging and spectroscopy of developmental disorders
Functional near-infrared spectroscopy (fNIRS) is a noninvasive and wearable imaging technique that assesses brain function and is suitable for studies of children and toddlers, especially those with neurodevelopmental disorders. Such measurements are based on local changes in the cerebral hemodynamic response associated with brain activity. NIR light (700–900 nm) can penetrate deep enough through tissue to probe the cortical region. The NIR absorption spectrum of tissue is sensitive to changes in the concentration of major tissue chromophores such as hemoglobin. Therefore, measurements of temporal variation in backscattered light can capture functionally evoked changes in the cortex to assess brain function. We are currently pursuing two general tracks of research involving fNIRS in the brain: the developmental trajectories of cognitive abilities and the evaluation of fNIRS using cognitive tasks that are used in functional magnetic resonance imaging (fMRI).
Click image to view.
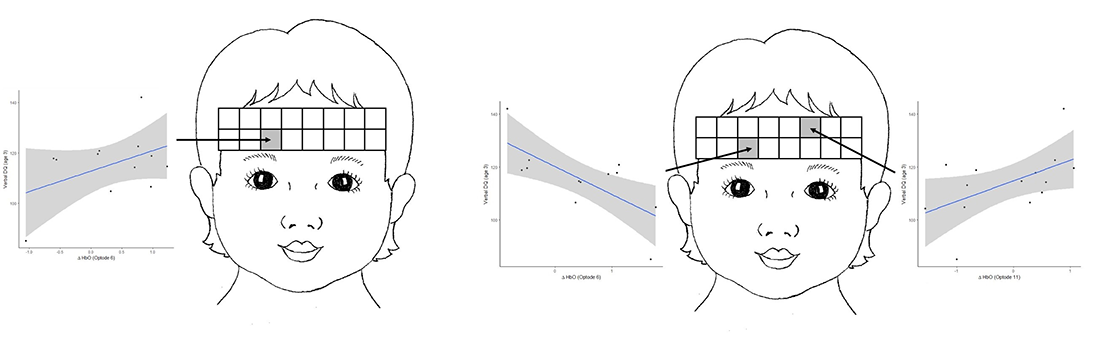
Figure 1. Developmental quotient (DQ) and age
A. Difference in HbO z-scores between meaningful gesture and non-meaningful gesture conditions predicts verbal developmental quotient at age three.
B. The difference in HbO z-scores between meaningful speech and meaningful gesture conditions predicts verbal DQ at age three.
In one line of research we use fNIRS to examine prefrontal cortical activation as it relates to developmental level in toddlers. Specifically, we examined brain activity in prefrontal regions in 24-month-old toddlers while they listened to speech sounds or watched gesture production, while we simultaneously recorded fNIRS in the prefrontal cortex (PFC). The stimuli allowed us to contrast brain activation across different types of communication and communicative intent. Our most recent publication demonstrated differential activation to gesture compared with speech stimuli, as well as differential activation to meaningful versus non-meaningful stimuli. Importantly, the differences in mean activation in the left PFC in response to meaningful gesture (when controlling for meaningless gesture) at age 2 predicted verbal ability at age 3 (Figure 1A). Differences in mean activation in response to meaningful speech compared with meaningful gesture at age 2 also predicted verbal ability at age 3 (Figure 1B). The findings may reflect potential biomarkers for aspects of language development.
In another study, we are using fNIRS combined with electroencephalography (EEG) to measure brain activity in the mirror-neuron network (MNN). The MNN is associated with the development of sophisticated social behaviors that emerge in typical infants. By modeling MNN development, we hope to uncover a sensitive measure of deviations in social communication development before clinical behavioral deficits can be detected. MNN activation has been indicated through mu rhythm suppression using EEG. Through a clinical protocol "Mirror neuron network dysfunction as an early biomarker of neurodevelopmental disorder" (18-CH-N001), we are now finalizing our adult pilot study (n= 52) to determine whether MNN activation can be elicited, using a motor observation and a simultaneous execution paradigm and EEG/fNIRS systems. Preliminary results show a strong negative correlation between mu rhythm suppression in EEG electrodes and fNIRS channels for action execution (Figure 2A). In addition, as hypothesized, the greatest correlations are found between EEG electrodes placed on central clusters (C3) and fNIRS channels placed on the left somato-sensorimotor regions, namely the left postcentral gyrus, left superior frontal gyrus, and left precentral gyrus (Figure 2B). We will further examine the synchronicity of these signals using more advanced machine-learning methods to examine how the features from both signals relate to each other and to help characterize brain function in the MNN. We will start recruiting typically developing infants (n=60) and infants at risk for developmental delays (n=60) from 9–12 months of age for the second phase of this project by January 2020. At-risk infants will be brought in again at 24 months of age to evaluate any deviations in their social communicative development. We will examine their developmental status at 24 months in relation to their initial neural data to determine whether MNN activation can predict developmental outcomes.
Click image to view.
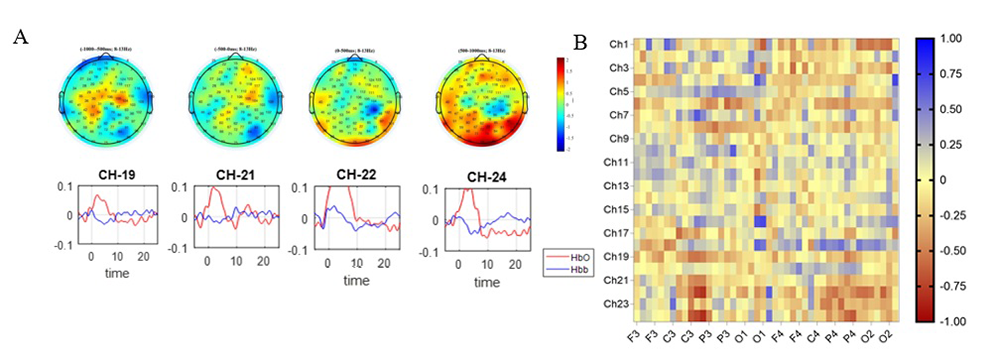
Figure 2. Mirror neuron network assessed with fNIRS and EEG
A, top. Topoplot of action execution between –1000 ms and +1000 ms of start action. A, bottom. Hemodynamic response function in channels recorded from channels placed over left postcentral gyrus, left superior frontal gyrus, and left precentral gyrus (channels 19, 21, 22, 24).
B. Heat map showing the correlations between the 2 signals. The greatest negative correlation is found between fNIRS channels placed over the left postcentral gyrus, left superior frontal gyrus, and left precentral gyrus and EEG electrodes placed on C3 and P3 clusters.
In a collaboration with Andrea Gropman, we are also examining brain function in patients with urea-cycle disorders (UCD). UCDs are a set of rare genetic disorders caused by the loss of those enzymatic activities (such as ornithine transcarbamylase deficiency [OTCD]) that convert ammonia to urea through the transfer of nitrogen. UCD often results in life-threatening hyperammonemia, resulting in a broad range of neurological impairments in working memory and executive function. We used fNIRS to measure brain activity in the PFC while patients performed a working memory task, namely a Stroop Interference task. Our results show that activation based on changes in HbO signal in left PFC is higher in controls than in UCD subjects. In addition, we used the concept of Hilbert Transform to calculate the instantaneous amplitude of total hemoglobin (HbO+HbR) in very low frequency band (VLF, <0.03 Hz) related to cerebral autoregulation. We applied this approach on a twin study of siblings with and without UCD. Our results, to be published soon, showed that, in the VLF region, the UCD sibling exhibited a lower degree of oscillation in instantaneous hemodynamic amplitude than did the control sibling during performance of working memory task.
We also used fNIRS to examine working memory in typically developing adults. To this end, we used an N-back working memory task, which provides a measure of mental workload. We tested 23 typically developing adults while fNIRS was recorded in the PFC. We compared the two task levels and the beginning and end of the 3-back task. Our findings indicated that there was a significant difference between the two task levels as a result of the task complexity. We also found a difference between the beginning and end of the 3-back task. We interpreted these findings as an improvement of subject’s performance resulting from the learning process of the brain. Thus, more correct answers were recorded, and less hemodynamic activation was seen, at the end of the 3-back task [Reference 1].
We conducted a study to examine neural activation during a 'go/no-go' behavior inhibition task that activates PFC areas. The go/no-go task was administered to 44 typically developing adults while fNIRS and heart rate were recorded. We found that fNIRS detected differences between baseline and the go/no-go task and could be a suitable alternative to fMRI in the evaluation of behavior inhibition. We are further analyzing the data to determine whether fNIRS measurements are related to individuals’ level of task performance or to more general measures of day-to-day behavior inhibition abilities. The analysis is ongoing, with the goal of submitting a manuscript by the end of 2019. We also drafted a manuscript examining heart-rate dynamics in relation to prefrontal activation, which will be submitted as part of a special issue in February 2020.
We used machine learning as a tool to identify features from fNIRS signal during a mental arithmetic task. We identified a set of features from the HbO signal to determine functionally connected channels during the task, along with latency differences among different brain regions. The features extracted to characterize functional connectivity during MA included the mean, variance, start, and slope of oxygenation activity. Three clusters corresponding to channels placed across the dorsolateral PFC (DLPFC), the temporal cortex, the posterior superior frontal cortex, and the ventrolateral PFC (VLPFC) were formed across different experimental runs for each participant, depicting functionally connected regions. The results indicated earlier activation of medial PFC than in the DLPFC, which might be related to the recruitment of retrieval-based techniques before the recruitment of additional cognitive skills such as a combination of decomposition, retrieval-based techniques, and updating processes. Our study shows that the technique can show temporal differences in activation across regions of the cortex and has the potential to distinguish between typical and impaired brain connectivity while performing a cognitive task [Reference 2].
Tissue characterization and function
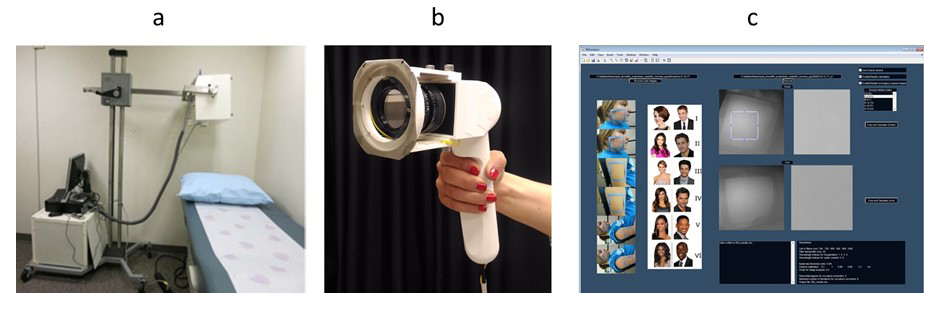
We are investigating photonic techniques to elucidate biomarkers for the diagnosis of disease or the assessment of treatment outcome across a variety of conditions. We are assessing facial plethora in Cushing’s syndrome (CS), as it was one of the earliest described clinical features of the disease. In collaboration with Constantine Stratakis, we quantified changes of facial plethora in CS as an early assessment of cure. We performed noninvasive multispectral NIR imaging on the right cheek of patients before and after surgery. Patients were defined as cured by postoperative measurements of plasma cortisol less than 3 (mcg/dl) and/or adrenocortical insufficiency, for which they received replacement therapy. Results indicate that a reduction in facial plethora after surgery, as evidenced by decrease in blood volume fraction, is correlated with the cure of CS. The first set of results were published in 2015. In our follow-up paper [Reference 4], we also showed that water content fraction could be used as a new biomarker of early cure in patients with CS. We recorded data for 29 new patients, and follow-up imaging was done for 26 patients. We developed and tested the new hand-held system that has improved performance over the existing portable system. We plan to use this system as a point-of-care imaging device. In brief, the new imager uses a high-resolution CMOS camera with on-chip filters. Images are acquired simultaneously at eight different near-infrared wavelengths (700–980 nm). Our graphical user interface (Figure 3c) now supports both portable and the hand-held multispectral imagers.
Click image to view.
Figure 3. Multispectral Imager from portable to point of care
Left. Portable multispectral imaging device.
Middle. New hand-held multispectral imager.
Right. Graphical user interface for data analysis.
Annually, about 15 million preterm infants are born in the world. Of these, about 1 million would die before the age of five because of complications resulting from their premature birth. Given that the high incidence of preterm birth (PTB) is partially the result of the lack of effective diagnostic modalities, methodologies are needed to determine the risk of PTB. We proposed a noninvasive tool based on polarized light imaging aimed at measuring the organization of collagen in the cervix. Cervical collagen has been shown to remodel with the approach of parturition. We used a full-field Mueller matrix polarimetric colposcope to assess and compare cervical collagen content and structure in nonpregnant and pregnant women in vivo. Local collagen directional azimuth was used and a total of eight cervices were imaged. In continued collaboration with Jessica Ramella-Roman on preterm pregnancy complications, we used the Preterm Imaging system based on colposcopy to characterize uterine cervix structure in a longitudinal study of low-risk and high-risk (i.e., prior PTB or a sonographic short cervix) patients. Polarization imaging is an effective tool to measure optical anisotropy in birefringent materials, such as the cervix's extracellular matrix, and to predict cervical ripening. For this reason, it has potential to predict preterm birth. Through our collaboration with Roberto Romero’s Branch and Ramella-Roman, we will test the system in a control population and those with PTB prevalence [Reference 5].
Placenta oxygenation from basics to point of care
Monitoring placenta oxygenation is critical to ensure a healthy pregnancy outcome. Abnormalities in placental oxygenation have been associated with preeclampsia, intrauterine growth restriction, fetal hypoxia, and cerebral palsy. Therefore, it is crucial to have a quantitative understanding of placental oxygenation. A placental oximeter should be light-weight, relatively small, battery-operated, and have wireless capability. Most importantly, the device must be relatively inexpensive, so it can be used in low-resource settings, where the most high-risk cases are to be expected. We fabricated a wearable oximeter using fNIRS technique for dynamic in vivo monitoring of anterior placental oxygenation. We intend to find the baseline placental oxygenation for normal pregnancies to standardize the oxygenation data across pregnancies and correlate pregnancy outcome with the placental oxygenation. In parallel, we are using a novel technique called Dynamic Full-Field Optical Coherence Tomography (DFFOCT) to study placental cell metabolism in vitro at physiologically relevant variations of oxygen levels.
Our oximeter device for in vivo studies is fast, non-invasive, and wearable, so that it allows continuous measurement of the oxygenation of the anterior placenta in a subject-friendly environment. The light-weight compact system is flexible and can thus be positioned at various abdominal locations for localized measurement of oxygenation. The NIRS device uses light in near-infrared region (760 and 840 nm) and consists of six source-detector pairs to simultaneously probe maternal and placental tissue. We investigated the efficiency of the device in separating oxygenation of the maternal and placental tissue while accounting for variations in melanin concentration and fat content. We developed a method to negate the effect of maternal tissues on the measurements using our multi-layer tissue model based on Monte Carlo simulation. The model includes the optical properties of skin, fat, uterus, and placental tissue. We further measured the optical properties of the placenta ex vivo using dual-wavelength LED sources with a higher resolution photodiode array unit built in-house, to calculate the attenuation coefficient (as a function of the scattering and absorption coefficients) based on the diffuse reflection curve from placental tissues. Using the above methods, we developed a system that includes parameters such as skin color and fat thickness in the calculation of oxygenation index.
In collaboration with Shad Deering and with Roberto Romero's Maternal-Fetal Medicine, Imaging, and Behavioral Development Affinity Group, we are testing our device in pilot studies. In our first pilot study, we are measuring the oxygenation of the placenta during the last trimester in normal pregnancies to establish the baseline placental oxygenation. Meanwhile, we are continuing to refine our data analysis software by incorporating anatomical data from subjects and ex vivo placental tissue measurements. By taking advantage of electronic miniaturization of spectroscopy, along with Artificial Intelligence for the classification of data between typical and atypical pregnancies, we expect to provide earlier detection of pregnancy complications, which can improve maternal and fetal health in the future.
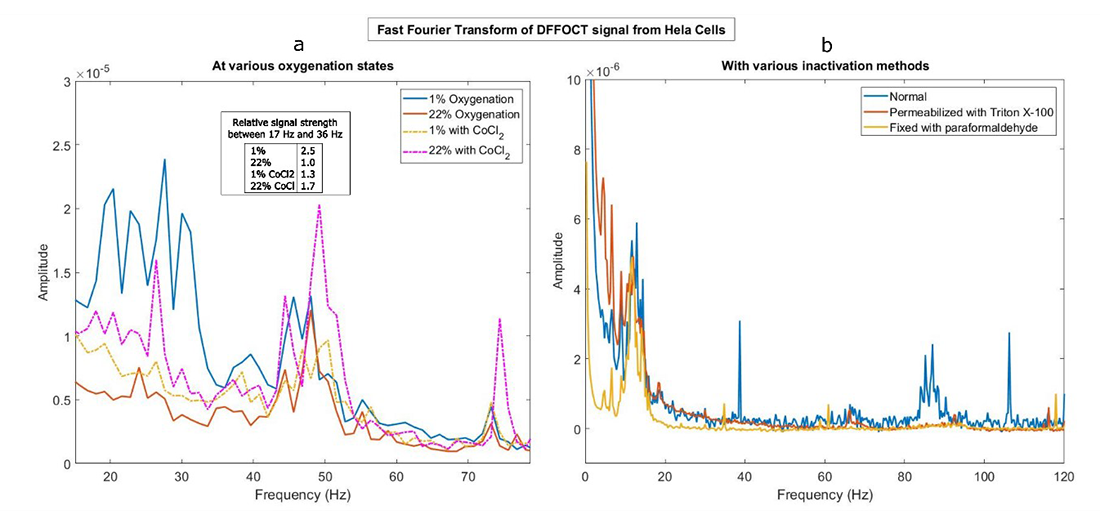
We established DFFOCT as a useful tool to detect the changes in intracellular activity as a function of ambient oxygenation. The in vitro experiments were performed on HeLa cells. DFFOCT signal from the cells grown on porous membrane was isolated using segmentation algorithms and analyzed using Fourier transform to understand the dynamic activity occurring within the cells. Signals from cells grown under hypoxia and physoxia (physiological oxygenation) show unique differences in the Fourier spectra under these conditions. Control experiments with cobalt chloride were used to mimic hypoxia. We also ran Western blots to confirm the effects of hypoxia on expression of the Hif-1α protein in the cells. Further, to identify the cellular activities that are responsible for DFFOCT signal, we inactivated cells in a variety of ways, such as para-formaldehyde fixation and treatment with Triton x100. The experiments show that loss of DFFOCT signal correlates with the treatment (Figure 4). Currently, we are investigating the cellular mechanisms affected by oxygenation by designing precise control experiments. This will help us use DFFOCT as a tool to assess activity of placental cells under altered oxygenation conditions.
Click image to view.
Figure 4. Dynamic full-field optical coherence tomography for cell dynamics
a. FFT spectra of HeLa cells grown under the following conditions: oxygenation of 22% (red), 1% (blue), 22% with CoCl2 (pink, dotted) and 1% with CoCl2 (yellow, dotted). Inset shows the normalized strength of the spectra in the range 17Hz to 36 Hz as integration (normalized with respect to 22% oxygenation).
b. FFT spectra of Hela Cells under three conditions: normal (blue), permeabilized with TritonX 100 (red), and fixed with 4% paraformaldehyde for 20 minutes (yellow).
Additional Funding
- Bench to Bedside Award 345 (2016): "Mirror neuron network dysfunction as an early biomarker of neurodevelopment" (Ongoing)
- Human Placenta Project-NICHD (2016) (Ongoing)
Publications
- Anderson AA, Parsa K, Geiger S, Zaragoza R, Kermanian R, Miguel H, Dashtestani H, Chowdhry FA, Smith E, Aram S, Gandjbakhche AH. Exploring the role of task performance and learning style on prefrontal hemodynamics during a working memory task. PloS One 2018;13(6):e0198257.
- Dashtestani H, Zaragoza R, Pirsiavash H, Knutson KM, Kermanian R, Cui J, Harrison JD Jr, Halem M, Gandjbakhche A. Canonical correlation analysis of brain prefrontal activity measured by functional near infra-red spectroscopy (fNIRS) during a moral judgment task. Behav Brain Res 2018;359:73-80.
- Khaksari K, Condy E, Millerhagen JB, Anderson AA, Dashtestani H, Gandjbakhche AH. Effects of performance and task duration on mental workload during working memory task. Photonics 2019;6(3):94.
- Afshari A, Keil M, Lyssikatos C, Belyavskaya E, Valdés N, Chowdhry FA, Parsa K, Ardeshirpour Y, Pursley R, Khare S, Kainerstorfer JM, Chittiboina P, Lodish MB, Mazzuchi TA, Gandjbakhche AH, Stratakis CA. Optical imaging technology: a useful tool to identify remission in Cushing disease after surgery. Horm Metab Res 2019;51(2):120-126.
- Chue-Sang J, Holness N, Gonzalez M, Greaves J, Saytashev I, Stoff S, Gomes J, Jung R, Gandjbakhche A, Chernomordik VV, Burkett G, Ramella-Roman JC. Use of Mueller matrix colposcopy in the characterization of cervical collagen anisotropy. J Biomed Opt 2018;23:1-9.
Collaborators
- Franck Amyot, PhD, Center for Neuroscience and Regenerative Medicine, Uniformed Services University of the Health Sciences, Bethesda, MD
- Claude Boccara, PhD, École Supérieure de Physique et de Chimie Industrielles, Paris, France
- Shad Deering, MD, Uniformed Services University of the Health Sciences, Bethesda, MD
- Andrea Gropman, MD, Children's National Health System, Washington, DC
- Sonia S. Hassan, MD, Wayne State University School of Medicine, Detroit, MI
- Jay Knutson, PhD, Laboratory of Molecular Biophysics, NHLBI, Bethesda, MD
- Maya Lodish, MD, Pediatric Endocrinology Inter-Institute Training Program, NICHD, Bethesda, MD
- Tom Pohida, MS, Division of Computational Bioscience, Center for Information Technology, NIH, Bethesda, MD
- Randall Pursley, Signal Processing and Instrumentation Section, CIT, NIH, Bethesda, MD
- Jessica C. Ramella-Roman, PhD, Florida International University, Miami, FL
- Roberto Romero-Galue, MD, Perinatology Research Branch, NICHD, Detroit, MI
- Dan Sackett, PhD, Division of Basic and Translational Biophysics, NICHD, Bethesda, MD
- Babak Shadgan, MD, MSc, PhD, University of British Columbia, Vancouver, Canada
- Constantine Stratakis, MD, D(med)Sci, Section on Endocrinology and Genetics, NICHD, Bethesda, MD
- Audrey Thurm, PhD, Pediatrics & Developmental Neuropsychiatry Branch, NIMH, Bethesda, MD
- Eric Wassermann, MD, Cognitive Neuroscience Section, NINDS, Bethesda, MD
Contact
For more information, email amir@helix.nih.gov or visit http://safb.nichd.nih.gov.


