Signaling and Secretion in Neuroendocrine Cells

- Stanko S. Stojilkovic, PhD, Head, Section on Cellular Signaling
- Aloa L. Dams, PhD, Visiting Fellow
- Yuta Moshimaru, PhD, Visiting Fellow
- Rafael M. Previde, PhD, Visiting Fellow
- Kosara Smiljanic, PhD, Visiting Fellow
- Srdjan J. Sokanovic, PhD, Visiting Fellow
- Kai Wang, PhD, Visiting Fellow
- Qing Chen, MD, Special Volunteer
We investigate cellular signaling cascades, gene expression, and hormone secretion in hypothalamic and pituitary cells, with a special emphasis on the interactions between plasma-membrane electrical events and receptor-controlled pathways. Specifically, we are addressing how these neuroendocrine cells use ion channels and G protein–coupled receptors as signaling platforms to efficiently process information. To this end, we characterize both native and recombinant receptors and channels that have been cloned from neuroendocrine cells. In the past, our work has focused on the role of inositol-trisphosphate receptors in the oscillatory calcium release of pituitary cells, the mechanism of periodic activation of these channels, and the complex mode of synchronization of calcium release from intracellular stores with electrical activity of cells. We also characterized voltage-gated channels expressed in neuroendocrine cells, the cell type–specific patterns of electrical activity and channels involved, the physiological relevance of such activity, and the crosstalk between G protein–coupled receptors and ion channels. More recently, we characterized ligand-gated receptor channels expressed in pituitary cells, including the ATP–gated P2X receptor channels. Our current work focuses on age-, sex-, and tissue structure–specific signaling, transcription, and secretion in the pituitary gland, the heterogeneity of secretory pituitary cells reflecting their embryonal and postnatal genesis, and cell type–specific exocytic pathways. We are also studying how the structural features of P2X receptors relate to the channels' functions and how plasma-membrane receptors and the intracellular signaling milieu affect channel activity.
Transcriptome profiles of rat anterior pituitary cells
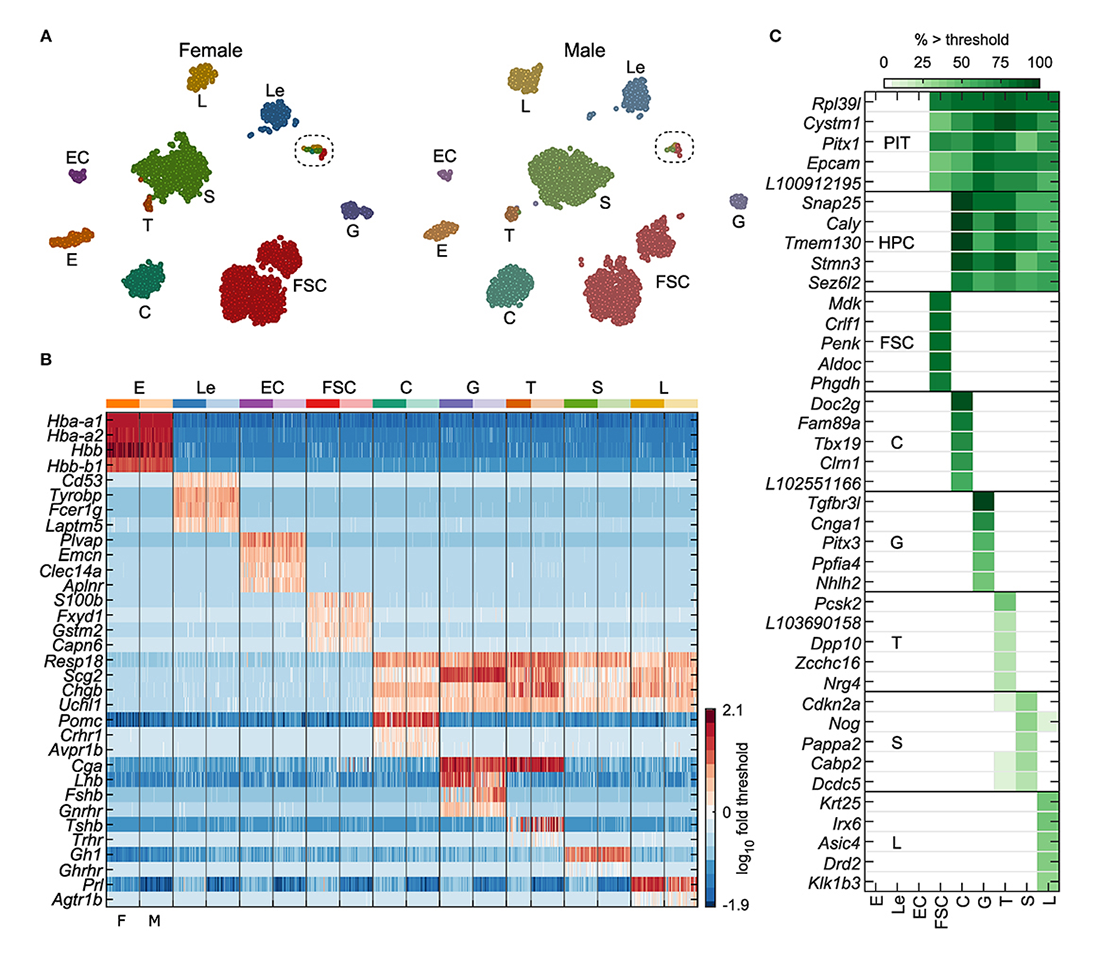
We continued our investigations on receptors and channels expressed in neuronal and endocrine cells and their roles in signaling, gene transcription, and hormone secretion. To gain a better understanding of the cell type-specific expression and role of these and other proteins in anterior pituitary cell functions and related disorders, we performed single-cell RNA sequencing on freshly dispersed cells from adult male and female rats. Our analysis, based on over 7,000 cells, confirmed the presence of folliculostellate cells (FSC) and hormone producing cells (HPC): corticotrophs, gonadotrophs, thyrotrophs, somatotrophs, and lactotrophs. Also recognized were endothelial and blood cells from the pituitary capillary network (Figure 1). We identified cell type–specific gene expression in HPCs and FSCs, examined cellular heterogeneity in these populations, and compared them with pituitary-nonspecific cell populations from portal blood capillaries.
Click image to view.
Figure 1. Identification of anterior pituitary cell types
A. tSNE map showing identified cell types for both female and male cells: erythrocytes (E), leukocytes (Le), endothelial cells (EC), folliculostellate cells (FSC), corticotrophs (C), gonadotrophs (G), thyrotrophs (T), somatotrophs (S), and lactotrophs (L). The results of marker-based classification, performed separately per sex, align very closely with the independent clustering done by tSNE.
B. Expression of genes used for cell-type classification in a random subsample of 50 cells per sex for each cell type, shown as log10 fold change relative to gene threshold. Columns alternate between female and male cells (F, M; bottom).
C.. Selected genes identified as specific markers or cell type–dominant genes, excluding classification genes. PIT: pituitary cells, comprising FSCs and HPCs. The gene name prefix LOC was shortened to L. Cell types E, Le, EC, and FSC were defined by expression greater than threshold of at least two of the four marker genes indicated per group. Hormone-producing cells (HPCs) comprised C, G, T, S, and L, all of which expressed at least two of Resp18, Scg2, Chgb, and Uchl1. Specific HPC types expressed additional genes as follows: C, at least one of Pomc, Crhr1, or Avpr1b; G, at least two of Cga, Lhb, Gnrhr, or Fshb; T, at least two of Cga, Tshb, or Trhr; S, Gh1 or Ghrhr; L, Prl or Agtr1b.
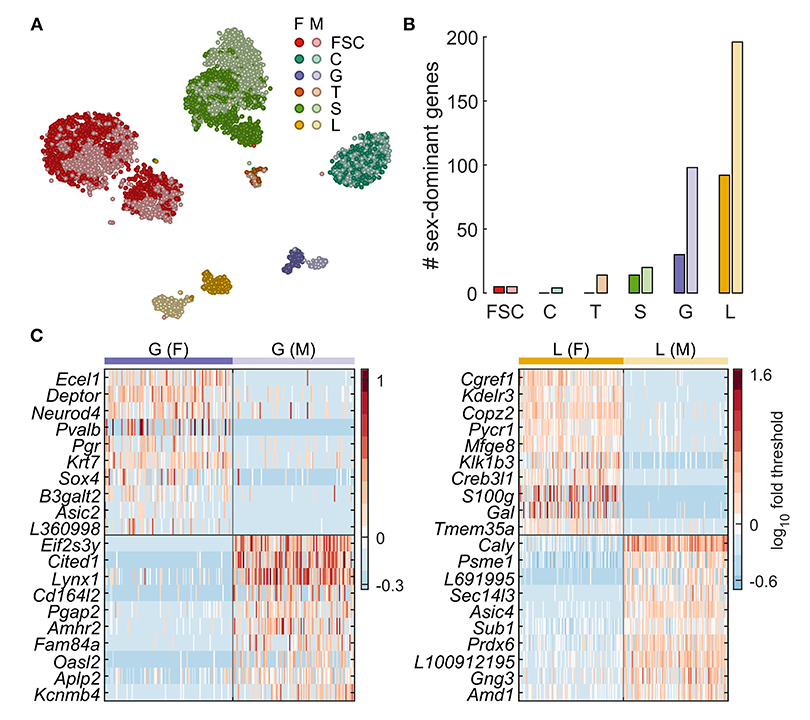
Comparison of males and females, the latter in the diestrus phase of their estrous cycle, allowed us to identify sex-specific gene expression profiles in pituitary-specific cell types. The t-distributed stochastic neighbor embedding maps shown in Figure 2 demonstrating clear sexual dimorphism in some cell types but not in others. This is evident for lactotrophs and gonadotrophs, both of which appear as two distinct clusters, whereas, for somatotrophs, a subset of female cells appears distinct from a mixed main cluster. FSCs also formed two clusters, but both clusters contained a mix of cells from both sexes, indicating that sexual dimorphism was not the cause of subclustering. The t-distributed stochastic neighbor embedding map also suggests very little sexual dimorphism in corticotrophs, based on uniform mixing of male and female cells in that cluster.
Click image to view.
Figure 2. Sexually dimorphic gene expression underlies lactotroph and gonadotroph subclustering.
A. Sex-specific clustering of pituitary cells shown as a tSNE plot. For a given cell type, females are indicated with a darker color.
B. Number of genes identified as dominantly expressed per sex for each cell type.
C. Expression of genes with the highest difference in % cells expressing more than threshold between sexes for gonadotrophs and lactotrophs. A random subsample of 100 female (left of vertical line) and 100 male cells (right of vertical line) are shown. F, female; M, male. The gene name prefix LOC was shortened to L.
To study the genetic relationship between FSCs and HPCs, we focused on two groups of genes suggested by our differential expression analysis: development and neuroendocrine marker genes. Out of 64 development-differentiation genes expressed in pituitary cells we identified 13 FSC–specific (including Prrx1, Prop1, and Sox9), 11 specific for one or more hormone-producing cell types (including Tbx19, Gata2, and Pou1f1), and 9 genes common to FSCs and at least one HPC type (including Pitx1, Pitx3, and Six1). Many neuroendocrine marker genes were widely expressed in HPCs; 28 were expressed in at least 80% of one or more cell type of this group. Among this gene group, Fuz, Gnas, Nptn, and Rtn1 were expressed in both cell types. Snap25, Snap91, Stmn3, Syt4, and Syt7 were well expressed in all HPC types and were identified as specific markers for this population of pituitary cells. Ndrg2 and Tagln were expressed dominantly and specifically in FSCs, respectively. In total, only 9 genes were FSC–specific, 47 were HPC–specific, and 14 were dominantly co-expressed by both FSC and HPC types. The enrichment in neuroendocrine genes related to exocytosis in HPCs is consistent with the physiological role of hormone secretion in these cells. Together, the findings suggest that HPCs and FSCs are either sister cells, i.e., cells of a common origin, or that a subset of FSCs are pituitary stem cells [Reference 1].
FSCs exhibit impressive transcriptome diversity, indicating their major roles in the production of endogenous ligands and detoxification enzymes, and organization of the extracellular matrix. Transcriptome profiles of HPCs also indicate contributions toward those functions. Among endogenous ligand genes, 15 genes were FSC–specific, including Anxa1, Cxcl12, Igfbp2, Mdk, and Penk; 18 genes were HPC–specific, including C1qtnf4 and Fgf9 in all HPCs, Inha, and Vegfa in gonadotrophs, and Bmp15 and Nmu in thyrotrophs; and 11 genes were common to FSCs and one or more HPC type, including Anxa7, Ccl27, Copa, and Cntn1. Among 79 detoxification enzyme genes expressed in pituitary cells, 20 were FSC–specific, six were common for FSCs and HPCs, and only seven genes were HPC–specific. Among 53 extracellular matrix genes expressed in pituitary cells, only five were FSC–specific, eight HPC-specific, and three common to pituitary cells. HPCs but not FSCs also express numerous genes encoding voltage-gated, ligand-gated, and other channels, clearly indicating their endocrine function. The findings point to complex interactions between HPCs and FSCs in the production of endogenous ligands and detoxification enzymes, organization of the extracellular matrix, and expression of cell-adhesion molecules [Reference 1].
Gating properties of purinergic receptor channels
Single-cell RNA sequencing also confirmed that all HPC types express ionotropic receptor channels activated by extracellular ATP, termed P2X4 channels, whereas gonadotrophs and folliculostellate cells express the P2X2 receptor gene. Both channels have unique properties. Our work on P2X2 receptors focused on the kinetics of receptor desensitization. The channels exhibit a slow desensitization during the initial ATP application and a progressive, calcium-dependent increase in rates of desensitization during repetitive stimulation. The pattern is observed in whole-cell recordings from cells expressing recombinant and native P2X2, but not in perforated-patched cells and in two-electrode voltage clamped oocytes. Addition of ATP, but not of ATPgS or GTP, to the pipette solution also abolishes progressive desensitization, whereas intracellular injection of apyrase facilitates receptor desensitization. Experiments with injection of alkaline phosphatase or addition of staurosporine and ATP to the intracellular solution suggest a role for a phosphorylation-dephosphorylation in receptor desensitization. Mutation of residues that are potential phosphorylation sites identified a critical role of the S363 residue of P2X2R in the intracellular ATP action. The findings indicate that the metabolic state of the cell can influence P2X2R gating [Reference 2].
We also used electrophysiology and recombinant channels to study in detail the antagonistic effect of 5-(3-bromophenyl)-1,3-dihydro-2H-benzofuro[3,2-e]-1,4-diazepin-2-one (5-BDBD) on ATP–induced currents mediated by the rat P2X4 receptor and compared its specificity with another rat P2X receptors. We found that 5-BDBD is a potent P2X4 receptor antagonist, with an IC50 in submicromolar concentration range. In contrast, 5-BDBD did not affect the ATP–induced P2X2a, P2X2bR, and P2X7 receptor current amplitude or the pattern of receptor desensitization when applied in a high micromolar concentration range. However, it partially inhibited the P2X1 and P2X3 receptor–gated currents. Moreover, we studied the effects of 5-BDBD in long-term potentiation experiments performed in rat hippocampal slices, with the finding that this antagonist can partially reduce long-term potentiation, a response that is believed to be mediated in part by endogenous P2X4 receptors. These results indicate that 5-BDBD could be used to study the endogenous effects of the P2X4 receptor in the central nervous system and that this antagonist can distinguish between this and other P2X receptors, when they are co-expressed in the same tissue [Reference 3].
Expression of small integrin-binding ligand, N-linked glycophosphoproteins (SIBLINGs) in gonadotrophs
The cell–extracellular matrix tridimensional network is critical for the proper functioning of all tissues, including anterior pituitary gland. In addition to proteoglycans and fibrous proteins, the extracellular matrix contains other proteins, including SIBLINGs, which are soluble, secreted proteins that can act as modulators of cell adhesion as well as autocrine and paracrine ligands for extracellular matrix receptors. Our single-cell RNA-seq analysis revealed that the pituitary gland expresses two SIBLING genes, Dmp1 (dentin matrix protein-1) and Spp1 (secreted phosphoprotein-1) encoding DMP1 and osteopontin proteins, respectively. Both genes were expressed exclusively in pituitary gonadotrophs, indicating that these cells have specific roles in the function of the cell–extracellular matrix tridimensional network of anterior pituitary gland. The qRT-PCR and immunocytochemical analyses confirmed that Spp1/osteopontin and Dmp1/DMP are expressed in a sex- and age-specific manner. The Spp1/osteopontin expression is higher in male gonadotrophs, whereas Dmp1/DMP1 is predominantly expressed in female gonadotrophs. Like Lhb, Fshb, and Gnrhr, the marker genes for gonadotrophs, Dmp1 expression is stimulated by gonadotropin-releasing hormone (GnRH). In contrast, the expression of Spp1 is not regulated by GnRH in vivo or in vitro. However, Spp1 expression increases progressively after pituitary cell dispersion in both female and male cultures. We speculate that gonadotrophs signal to other pituitary cell types about changes in the structure of pituitary cell-matrix network by osteopontin and DMP1, a function consistent with the role of these secretory proteins in postnatal tissue remodeling, extracellular matrix reorganization after injury, and tumorigenesis. Our ongoing work focuses on secretion of these two proteins by gonadotrophs under different experimental paradigms, on characterization of their receptors, integrins, and CD44, within secretory and non-secretory anterior pituitary cells and their signaling pathways, and on their function in pituitary gland [Reference 4].
Continuous GnRH treatment blocks Fshb but not Lhb expression.
The pulsatile pattern of GnRH secretion is critical for proper gonadotropin synthesis and release, and continuous GnRH infusion leads to a marked reduction in blood gonadotropin levels. However, the mechanism of downregulation of gonadotropin release by continuous GnRH treatment has not been clarified. Our ongoing experiments focus on the expression profile of gonadotropin subunit genes Cga, Lhb, and Fshb and of Gnrhr in rat pituitary in vitro and in vivo to clarify their expression profiles in the absence or continuous presence of GnRH. Figure 1 shows that the genes are expressed in pituitary gonadotrophs but not in other pituitary cell types. Culturing mixed populations of anterior pituitary cells in GnRH–free conditions downregulated Fshb, Cga, and Gnrhr expression, whereas continuous treatment with GnRH receptor agonists upregulated Cga expression progressively but Gnrhr and Fshb expression transiently, accompanied with a prolonged blockade of Fshb but not of Gnrhr expression. In contrast, Lhb expression was relatively insensitive to loss of endogenous GnRH and continuous treatment with GnRH agonists, probably reflecting the status of Egr1 and Nr5a1 expression. Similar patterns of responses were observed in vivo after administration of a GnRH agonist. However, continuous treatment with GnRH stimulated luteinizing hormone (LH) secretion in vitro and in vivo, leading to decrease in LH cell content despite high basal Lhb expression. The data suggest that blockade of Fshb expression and depletion of the LH secretory pool are two major factors accounting for weakening of the gonadotroph secretory function during continuous GnRH treatment [Reference 5].
Publications
- Fletcher PA, Smiljanic K, Maso Prévide R, Iben JR, Li T, Rokic MB, Sherman A, Coon SL, Stojilkovic SS. Cell type- and sex-dependent transcriptome profiles of rat anterior pituitary cells. Front Endocrinol (Lausanne) 2019;10:623.
- Rokic MB, Castro P, Leiva-Salcedo E, Tomic M, Stojilkovic SS, Coddou C. Opposing roles of calcium and intracellular ATP on gating of the purinergic P2X2 receptor channel. Intern J Mol Sci 2018;19:e1161.
- Coddou C, Sandoval R, Hevia MJ, Stojilkovic SS. Characterization of the antagonist actions of 5-BDBD at the rat P2X4 receptor. Neurosci Lett 2019;690:219-224.
- Bjelobaba I, Janjic MM, Prévide RM, Abebe D, Kucka M, Stojilkovic SS. Distinct expression patterns of osteopontin and dentin matrix protein 1 genes in pituitary gonadotrophs. Front Endocrinol (Lausanne) 2018;10:248.
- Janjic MM, Previde RM, Fletcher PA, Sherman A, Smiljanic K, Abebe D, Bjelobaba I, Stojilkovic SS. Divergent expression patterns of pituitary gonadotropin subunit and GnRH receptor genes to continuous GnRH in vitro and in vivo. Sci Rep 2019;9(1):20098.
Collaborators
- Claudio Coddou, PhD, Faculty of Medicine, Universidad Católica del Norte, Coquimbo, Chile
- Patrick A. Fletcher, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
- Arthur Sherman, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
Contact
For more information, email stankos@helix.nih.gov or visit https://neuroscience.nih.gov/Faculty/Profile/stanko-stojilkovic.aspx or http://irp.nih.gov/pi/stanko-stojilkovic.


