Physiology, Psychology, and Genetics of Obesity

- Jack A. Yanovski, MD, PhD, Head, Section on Growth and Obesity
- Miranda Broadney, MD, MPH, Acting Director, Pediatric Endocrinology Fellowship Program
- Andrew P. Demidowich, MD, Staff Clinician
- Lisa Shank, PhD, Research Collaborator
- Sheila M. Brady, RN, FNP, Nurse Practitioner
- Robin Roberson, MS, Technician
- Deborah Altman, BA, Guest Researcher
- Meghan Byrne, MA, Guest Researcher
- Megan Parker, MS, Guest Researcher
- Sarah Russell, MA, Guest Researcher
- Taylor N. Swanson, BA, Guest Researcher
- Tusharkumar Patel, PhD, Visiting Fellow
- Elizabeth K. Altman, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Kaitlin Ballenger, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Nejla Ghane, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Michael Jayson, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Jordan Levine, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Eliana Ramirez, BA, Postbaccalaureate Intramural Research Training Award Fellow
- Sarah Rubin, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Risha Sheni, BS, Postbaccalaureate Intramural Research Training Award Fellow
- Nicol Tugarinov, BS, Postbaccalaureate Intramural Research Training Award Fellow
The prevalence of overweight and obesity in children and adults has tripled during the past 40 years, an alarming rise in body weight that has likely occurred because the current environment affords easy access to calorie-dense foods and requires less voluntary energy expenditure. However, such an environment leads to obesity only in those individuals whose body weight–regulatory systems are not able to control body adiposity with sufficient precision in our high calorie/low activity environment, suggesting that there are subgroups in the U.S. with a uniquely high susceptibility to weight gain under the prevailing environmental conditions. Our primary goal is to elucidate the genetic underpinnings of the metabolic and behavioral endophenotypes that contribute to the development of obesity in children. Using our unique longitudinal cohorts of children at risk for adult obesity, who have undergone intensive metabolic and behavioral phenotyping, we examine genetic and phenotypic factors predictive of progression to adult obesity in children who are in the "pre-obese" state, allowing characterization of phenotypes unconfounded by the impact of obesity itself. Once they are identified as linked to obesity, we study intensively genetic variants that impair gene function. We expect that these approaches will improve our ability to predict which children are at greatest risk for obesity and its comorbid conditions and will lead to more targeted, etiology-based prevention and treatment strategies for pediatric obesity.
Genetic factors important for childhood body weight regulation
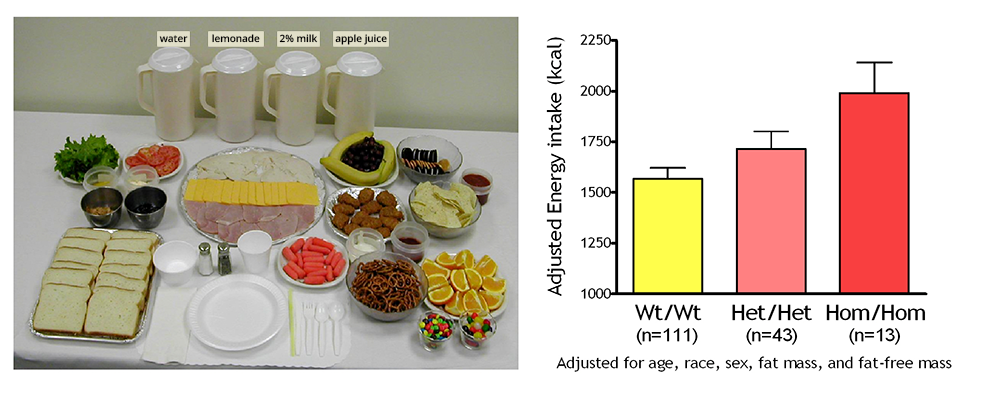
Click image to view.
Figure 1. Energy intake studied by using free-access buffet meals of palatable foods
Children homozygous for two polymorphisms in the MC3R gene (Hom/Hom) consumed more at the buffet than heterozygotes (Het/Het) or than those with wild-type MC3R (Wt/Wt).
To identify gene variants affecting body composition, we have been examining polymorphisms in genes involved in the leptin signaling pathway. Genes include the leptin receptor (LEPR), genes that appear to alter leptin receptor signal transduction such as those that are part of the BBSome (an octameric protein complex component of the basal body involved in trafficking cargos to the primary cilium), and those encoding proopiomelanocortin (POMC), the melanocortin 3 receptor (MC3R), the melanocortin 4 receptor (MC4R), and brain-derived neurotrophic factor (BDNF). We are currently studying a variant MC3R that is associated with adiposity in children and adults [Reference 1] and appears to have functional significance for MC3R signal transduction. Children and adults who were homozygous variant for both C17A and G241A polymorphisms have significantly greater fat mass and higher plasma levels of insulin and leptin than unaffected or heterozygous children and appear to eat more at laboratory test meals (Figure 1). In vitro studies subsequently found that signal transduction and protein expression were significantly lower for the double mutant MC3R. Our ongoing studies attempt to understand the mechanisms by which these sequence alterations may affect body weight. We therefore developed transgenic 'knock-in' mice expressing the human wild-type and human double-mutant MC3R. Using homozygous knock-in mouse models replacing murine Mc3r with wild-type human (MC3RhWT/hWT) and double-mutant (C17A+G241A) human (MC3RhDM/hDM) MC3R, we found that MC3RhDM/hDM mice have greater weight and fat mass (Figure 2), increased energy intake and feeding efficiency, but reduced length and fat-free mass compared with MC3RhWT/hWT. MC3RhDM/hDM mice do not have increased inflammatory cell infiltration of adipose tissue or greater expression of inflammatory markers, despite their greater fat mass. Serum adiponectin is increased in MC3RhDM/hDM mice and in MC3RhDM/hDM human subjects (Figure 2). MC3RhDM/hDM bone- and adipose tissue–derived mesenchymal stem cells (MSCs) differentiate into adipocytes that accumulate more triglyceride than MC3RhWT/hWT MSCs. MC3RhDM/hDM thus impacts nutrient partitioning to generate increased adipose tissue that appears metabolically healthy. The data thus confirm the importance of MC3R signaling in human metabolism and suggest a previously unrecognized role for the MC3R in adipose tissue development. Ongoing studies continue to improve our understanding of the phenotype of the mice. We are investigating a novel role for the MC3R in regulating hepatic autophagy, the role of MC3R in stem cell fate, and how variations in Mc3r may alter signaling of several downstream signaling pathways. Using tissue-specific knockout- and reactivation models, we are also studying the importance of hepatic and adipose tissue MC3R for whole body homeostasis.
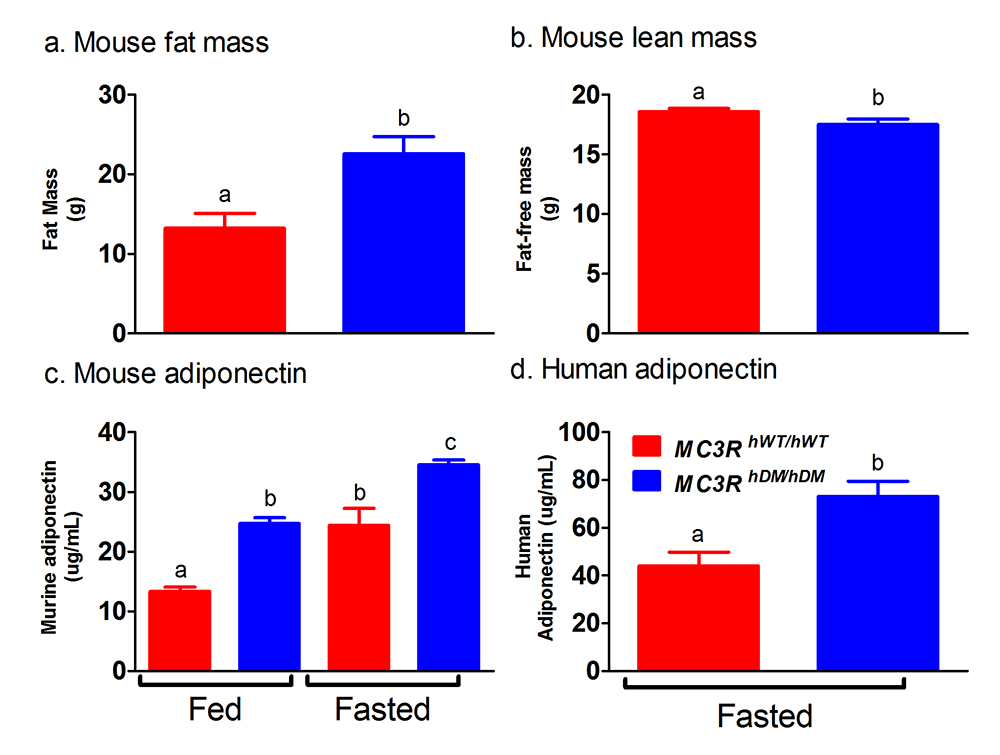
Click image to view.
Figure 2. Studies of a human MC3R variant containing two naturally occurring polymorphisms
The variant is associated with pediatric-onset obesity. We found that mice whose Mc3r was replaced by human versions of the gene were obese when they expressed the double-mutant gene (MC3RhDM/hDM) with greater fat mass (a) and lower fat-free mass (b), but surprisingly greater adiponectin concentrations (c) than mice with the normal human MC3R (MC3RhWT/hWT). Humans with the double-mutant receptor also showed greater adiponectin (d).
Physiology, metabolism, and psychology of childhood body weight regulation
Our studies are directed at understanding the physiological, psychological, and metabolic factors that place children at risk for undue weight gain. As part of these studies, we examined how best to measure eating-related psychopathology, insulin sensitivity, changes in body composition, energy intake, and energy expenditure in children, and we studied the short- and long-term stability of the components of metabolic syndrome. We found that leptin is an important predictor of weight gain in children: those with high leptin gain even more weight when followed longitudinally. We also documented that hyperinsulinemia is positively related to energy intake in nondiabetic, obese children, leading to treatment studies to reduce hyperinsulinemia. We also examined the relationship between depressive symptomatology and insulin resistance in children and adolescents, finding strong associations both cross-sectionally and prospectively between depressive symptoms and insulin resistance independent of body weight. Such associations suggest mechanisms whereby insulin resistance may contribute to excessive weight gain in children and have informed some of our treatment approaches to pediatric obesity (described below).
Our evaluations concentrating on binge-eating behaviors in children suggest that such behaviors also are associated with adiposity in children and abnormalities in metabolism. We found that binge-eating behaviors may predict future weight gain in children at risk for obesity. Thus children reporting binge-eating behaviors, such as loss of control over eating, gained, on average, 2.4 kg more weight per year than non-binge-eating children. Our data also suggest that children endorsing binge eating consume more energy during meals. Actual intake during buffet meals averaged 400 kcal more in children with binge eating, but, despite their greater intake, such children reported shorter-lived satiety than children without binge-eating episodes. The ability to consume large quantities of palatable foods, especially when coupled with decreased subsequent satiety, may play a role in the greater weight gain found in binge-eating children. Among cohorts of lean and obese youth, we demonstrated that youth with LOC (loss of control) eating have higher serum leptin and are at significantly greater risk for worsening of components of the metabolic syndrome than those without LOC episodes, even after adjusting for adiposity and other relevant covariates. Our data also suggest that anxiety symptoms may interact with LOC eating to become an important co-factor for excessive weight gain among children. The data further suggest that interventions targeting disordered eating behaviors may be useful in preventing excessive fat gain in children prone to obesity and have led to trials of preventative strategies related to binge eating. Because binge eating appears to be a heritable trait, we also initiated studies to investigate possible genetic factors linked to LOC over eating.
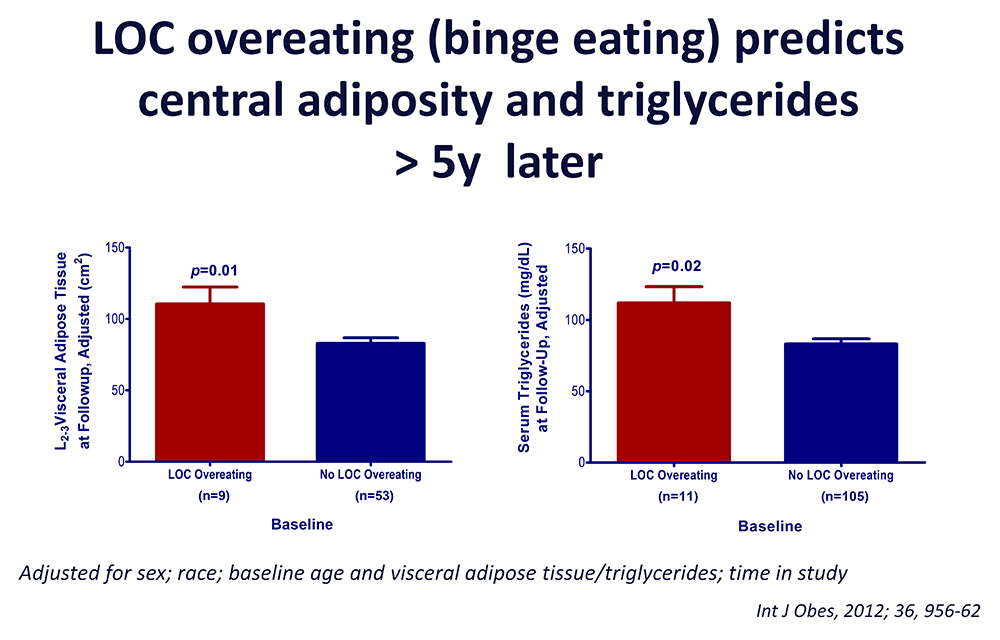
Click image to view.
Figure 3. Loss of control (LOC) eating and metabolic complications in a longitudinal study
On average (±SE), children who engaged in binge eating at baseline had more visceral adipose tissue at L2-3 intervertebral space at follow-up than children who did not engage in binge eating at baseline, adjusting for sex, race, baseline age, baseline visceral adipose tissue at L2-3, and time in study (P = 0.01). On average (±SE), children who engaged in binge eating at baseline had higher follow-up triglycerides than children who did not engage in binge eating at baseline, adjusting for sex, race, baseline age, body mass index (kg/m2), baseline triglycerides, and time in study (P = 0.02).
In order to determine the factors that are most important for development of the complications of obesity in youth, we study normal weight children and adolescents, children who are already obese, and the children of obese parents who are not obese. We examine body composition, leptin concentration, metabolic rate, insulin sensitivity, glucose disposal, energy intake at buffet meals, and genetic factors believed to regulate metabolic rate and body composition. Psychological and behavioral factors, such as propensity to engage in binge-eating behavior (Figure 3), and sleep [Reference 2] are also studied. Children are being followed longitudinally into adulthood. In two protocols, we study actual food consumption of children during meals, to elucidate differences in the calorie and macronutrient content of meals and the circulating hormones related to hunger and satiety in those who either endorse binge-eating behaviors or report no such behaviors. We found that eating in the absence of physiological hunger is a replicable trait that appears linked to obesity. We also investigated the role of sedentary behaviors, such as television watching, as a factor that alters metabolism. In a randomized, controlled, crossover trial (Figure 4), we found that glucose homeostasis was markedly improved in children with overweight or obesity who engaged in moderate activity for just three minutes every half hour, versus remaining sedentary [Reference 3]. A new study extends these observations over a longer intervention period. We hypothesize that differences in these factors will predict the development of obesity in the populations studied and may be of great importance in developing rational approaches for the prevention and treatment of obesity in the diverse US population.
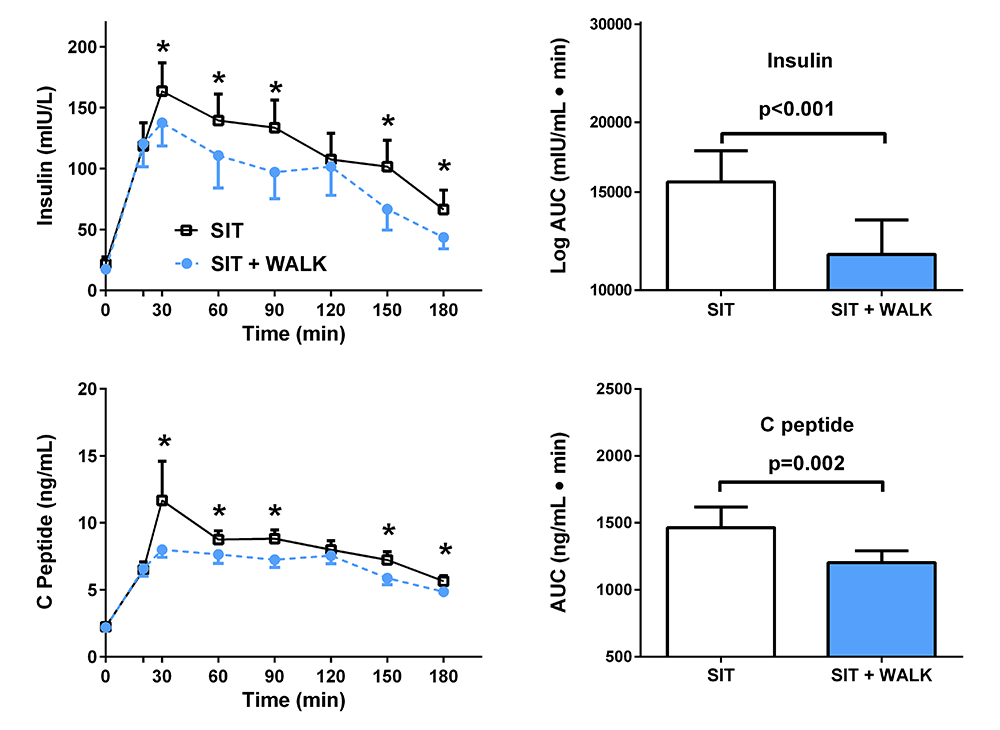
Click image to view.
Figure 4. Effect of short, moderate intensity walking breaks on children’s glucose tolerance
Children with overweight or obesity who walked for three minutes every 30 minutes (blue) had lower insulin and C-peptide concentrations during an oral glucose tolerance test than when they sat uninterrupted for three hours (black).
Treatment of obesity and the co-morbid conditions associated with obesity
Given the rapid increase in the prevalence of obesity, the development of treatments for obesity in children and adults is urgently needed, yet current pharmacologic approaches are extremely limited for both children and adults. In several clinical protocols, we examined approaches for the prevention and treatment of excessive body weight. We completed a randomized controlled trial to examine the mechanism by which metformin may affect the body weight of younger children who have hyperinsulinemia and are therefore at risk for later development of type 2 diabetes. Compared with placebo-treated children, those randomized to metformin significantly decreased BMI, BMI-Z score, and body fat mass. Serum glucose and HOMA-IR also decreased significantly more in metformin-treated than in placebo-treated children. We also published studies of the pharmacokinetics of metformin and how polymorphisms in enzymes affecting metformin clearance impact weight change. Mean population apparent clearance (CL/F) was 68.1 L/h, and mean apparent volume of distribution (V/F) was 28.8 L. Body weight was a covariate of CL/F and V/F. Estimated glomerular filtration rate was a significant covariate of CL/F. Carriers of an SLC22A1 variant (SLC22A1 is a protein required for metformin uptake by cells) had significantly smaller reductions in percentage of total trunk fat after metformin therapy. The median change in trunk fat was –2.20% (–9.00% to 0.900%) and –1.20% (–2.40% to 7.30%) for the SLC22A1 wild-type subjects and variant carriers, respectively.
A third study examined prevention of weight gain using interpersonal therapy (IPT) versus a control health education program (HE) in adolescents reporting loss of control eating behaviors. At three-year follow-up, baseline social-adjustment problems and trait anxiety significantly moderated outcome. Among girls with high self-reported baseline social-adjustment problems or anxiety, IPT, compared with HE, was associated with the steepest declines in BMIz. For adiposity, girls with high or low anxiety in HE and girls with low anxiety in IPT experienced gains, while girls in IPT with high anxiety stabilized. Parent reports yielded complementary findings. The results have stimulated ongoing research to examine how anxiety may stimulate energy intake. We also published preliminary data from a fourth study examining IPT approaches in younger children, finding good tolerability for such a program.
A fifth study examined whether reducing depressive symptoms could ameliorate insulin resistance in adolescents at-risk for type 2 diabetes. Among 78 girls with greater (moderate) baseline depressive symptoms, those in cognitive behavioral therapy (CBT) developed significantly lower 2-hr insulin than those in HE. Additional metabolic benefits of CBT were seen for this subgroup between post-treatment and 1-year follow-up in post-hoc analyses.
An ongoing investigation, based on prior lab data that found links between attentional biases to high-palatability foods in children with obesity, studies whether adolescents’ attentional biases can be retrained. We also initiated a translational trial studying the effects of modulation of the leptin signaling pathway with the melanocortin agonist setmelanotide in patients with proximal signaling defects such as PCSK1 (an enzyme that processes prohormones) insufficiency and in patients with Bardet-Biedl syndrome. Initial data support the hypothesis that increasing activity at melanocortin receptors reduces food intake in people with monogenic disorders affecting the proximal leptin pathway. Most recently, we initiated another study of specific pharmacotherapy for patients with Prader-Willi syndrome using diazoxide. These latest trials are examples of precision medicine approaches [Reference 4] to treat obesity.
We also recently completed a novel randomized controlled pilot trial of colchicine to ameliorate the inflammation of obesity and thus improve its complications [Reference 5]. Adults with obesity and metabolic syndrome, but who did not have diabetes, were randomized to colchicine 0.6 mg or placebo capsules twice daily for three months. Compared with placebo, colchicine significantly reduced C-reactive protein and erythrocyte sedimentation rate. The changes in homeostatic model assessment of insulin resistance, fasting insulin, and glucose effectiveness suggested significant metabolic improvements in the colchicine-treated versus placebo group. These results suggest a larger, adequately powered study should be conducted to determine whether colchicine improves insulin resistance and other measures of metabolic health in at-risk individuals.
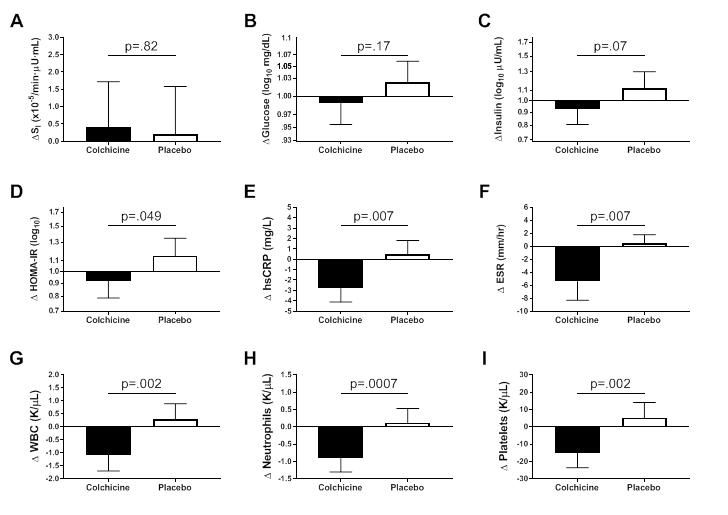
Click image to view.
Figure 5. Effects of colchicine on inflammatory and metabolic measures
Metabolic and inflammatory changes after three months of study medication in participants randomized to colchicine (N=21) or placebo (N=19).
A. Insulin sensitivity (SI).
B. Fasting glucose.
C. Fasting insulin.
D. Homeostasis Model Assessment of Insulin Resistance (HOMA-IR).
E. High sensitivity C-reactive protein (hsCRP).
F. Erythrocyte sedimentation rate (ESR).
G. White blood cell count (WBC).
H. Neutrophil count.
I. Platelet count. Data are presented as mean ± SEM.
Additional Funding
- NIH Clinical Center/ORD "Bench-to-Bedside" Award: Melanocortin agonist to bypass leptin resistance of Bardet-Biedl syndrome. 2017–2018
- Rhythm Pharmaceuticals, Inc.: Setmelanotide (RM-493; Rhythm Pharmaceuticals, Inc.) phase 2 open-label treatment trials in patients with rare genetic disorders of obesity. 2017–2020
- Soleno Therapeutics, Inc. Grant support to fund an RCT testing a diazoxide choline sustained release tablets in patients with Prader-Willi syndrome and hyperphagia. 2018–2020
- NICHD Division of Intramural Research Director's Investigator Award, 2018–2019
Publications
- Demidowich AP, Parikh VJ, Dedhia N, Branham RE, Madi SA, Marwitz SE, Roberson RB, Uhlman AJ, Levi NJ, Mi SJ, Jun JY, Broadney MM, Brady SM, Yanovski JA. Associations of the melanocortin 3 receptor C17A + G241A haplotype with body composition and inflammation in African-American adults. Ann Hum Genet 2019;83:355-360.
- Mi SJ, Kelly NR, Brychta RJ, Grammer AC, Jaramillo M, Chen KY, Fletcher LA, Bernstein SB, Courville AB, Shank LM, Pomeroy JJ, Brady SM, Broadney MM, Tanofsky-Kraff M, Yanovski JA. Associations of sleep patterns with metabolic syndrome indices, body composition, and energy intake in children and adolescents. Pediatr Obes 2019;14:e12507.
- Broadney MM, Belcher BR, Berrigan DA, Brychta RJ, Tigner IL, Jr, Shareef F, Papachristopoulou A, Hattenbach JD, Davis EK, Brady SM, Bernstein SB, Courville AB, Drinkard BE, Smith KP, Rosing DR, Wolters PL, Chen KY, Yanovski JA. Effects of interrupting sedentary behavior with short bouts of moderate physical activity on glucose tolerance in children with overweight and obesity: a randomized crossover trial. Diabetes Care 2018;41:2220-2228.
- Yanovski SZ, Yanovski JA. Toward precision approaches for the prevention and treatment of obesity. JAMA 2018;319:223-224.
- Demidowich AP, Levine JA, Onyekaba GI, Khan SM, Chen KY, Brady SM, Broadney MM, Yanovski JA. Effects of colchicine in adults with metabolic syndrome: a pilot randomized controlled trial. Diabetes Obes Metab 2019;21:1642-51.
Collaborators
- David Allison, PhD, School of Public Health, Indiana University, Bloomington, IN
- Jeffrey Baron, MD, Section on Growth and Development, NICHD, Bethesda, MD
- Karen Berman, PhD, Section on Integrative Neuroimaging, NIMH, Bethesda, MD
- Kong Chen, PhD, Clinical Endocrinology Branch, NIDDK, Bethesda, MD
- Ross Crosby, PhD, Director of Biomedical Statistics, Neuropsychiatric Research Institute, Fargo, ND
- Joel Elmquist, DVM, PhD, UT Southwestern Medical Center, Dallas, TX
- I. Sadaf Farooqi, MD, Cambridge Institute for Medical Research, Cambridge, United Kingdom
- Oksana Gavrilova, PhD, Mouse Metabolism Core Laboratory, NIDDK, Bethesda, MD
- Joan C. Han, MD, Le Bonheur Children’s Hospital, Memphis, TN
- Robert Haws, MD, Marshfield Clinic Research Institute, Marshfield, WI
- Steven B. Heymsfield, MD, Pennington Biomedical Research Center, Baton Rouge, LA
- Michael Jensen, MD, Mayo Clinic, Rochester, MN
- Rudolph L. Leibel, MD, Columbia University College of Physicians and Surgeons, New York, NY
- Sergey Leikin, PhD, Section on Physical Biochemistry, NICHD, Bethesda, MD
- Jennifer Lippincott-Schwartz, PhD, HHMI Janelia Research Campus, Ashburn, VA
- Cara Olsen, PhD, Uniformed Services University of the Health Sciences, Bethesda, MD
- Vipul Periwal, PhD, Laboratory of Biological Modeling, NIDDK, Bethesda, MD
- Daniel Pine, MD, Section on Development and Affective Neuroscience, NIMH, Bethesda, MD
- Douglas Rosing, MD, Cardiac Consultation Service, NHLBI, Bethesda, MD
- Peter J. Schmidt, MD, Section on Behavioral Endocrinology, NIMH, Bethesda, MD
- Lauren B. Shomaker, PhD, University of Colorado, Boulder, CO
- Eric Stice, PhD, Oregon Research Institute, Eugene, OR
- Marian Tanofsky-Kraff, PhD, Uniformed Services University of the Health Sciences, Bethesda, MD
- B. Timothy Walsh, PhD, Columbia University College of Physicians and Surgeons, New York, NY
- Denise E. Wilfley, PhD, Washington University School of Medicine, St. Louis, MO
- Joshua Zimmerberg, MD, PhD, Section on Integrative Biophysics, NICHD, Bethesda, MD
Contact
For more information, email yanovskj@mail.nih.gov or visit http://sgo.nichd.nih.gov.


