Three-Dimensional Organization of the Genome as a Determinant of Cell-Fate Decisions

- Pedro Rocha, PhD, Head, Unit on Genome Structure and Regulation
- Shreeta Chakraborty, PhD, Postdoctoral Fellow
- Joyce J. Thompson, PhD, Postdoctoral Visiting Fellow
- Ariel Eraso, BSc, Postbaccalaureate Intramural Research Training Award Fellow
- Sarah K. Frail, BSc, Postbaccalaureate Intramural Research Training Award Fellow
- Samuel Hernandez, BA, Postbaccalaureate Fellow
- Daniel Lee, BSc, Postbaccalaureate Fellow
- James Wang, Summer Student
Our lab is interested in understanding cell-lineage differentiation, gene regulation, and how non-coding DNA elements and the 3D architecture of chromosomes contribute to these processes during development and disease. We are also interested in early mammalian development as a system in which to decipher how cells make lineage decisions and how gene-regulatory networks are established.
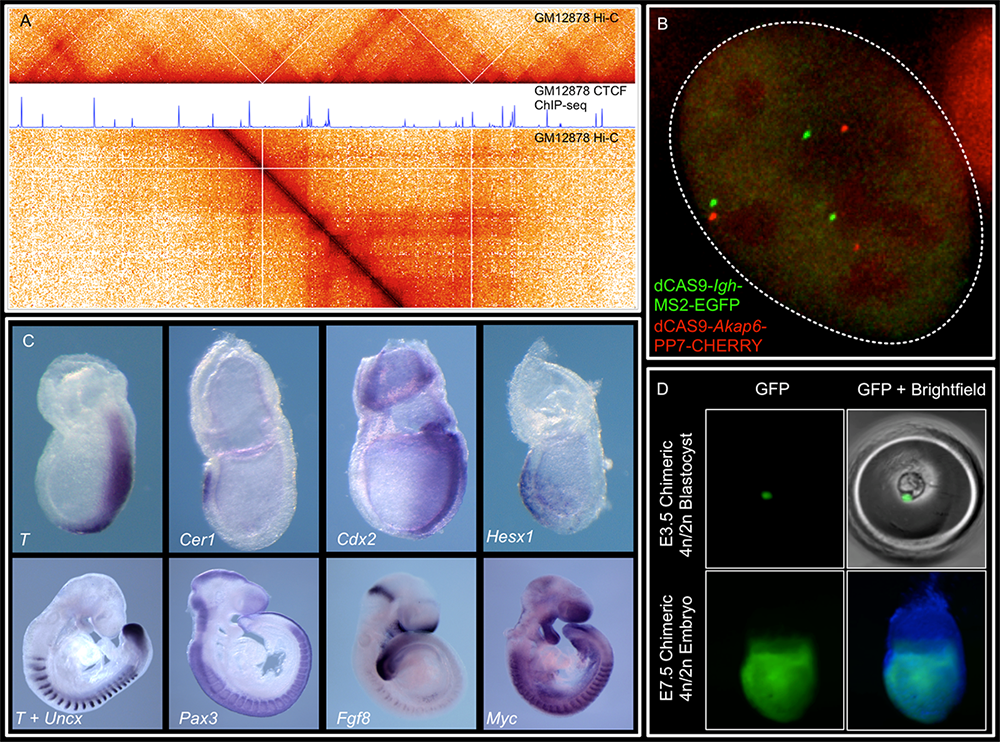
Click image to view.
Figure 1. Representative image of the lab's research
We combine imaging techniques in both fixed and living cells with sequencing-based genomic techniques that assess DNA–DNA interactions.
A. Hi-C and CTCF ChIP-seq of GM1278 cells, which allow characterization of chromatin structure and identification of binding sites of an important architectural protein.
B. dCAS9 MCP-EGFP and PCP-CHERRY live imaging of the Igh and Akap6 loci. The mouse embryo is an unparalleled system in mammalian biology for understanding how tissue-specific gene expression is achieved.
C. Whole mount in situ hybridization for patterning markers in mid and late gastrulating embryos
D. Tetraploid aggregation with GFP ES (embryonic stem) cells allows generation of fully ES–cell derived embryos.
Eukaryotic cells need to deal with the biophysical constraints of packaging two meters of DNA inside a tiny nucleus (2–10 microns) and still retain the ability to access both its coding and non-coding elements to precisely orchestrate gene expression programs. Research over the past decade has begun to elucidate the mechanisms through which DNA condensation and organization in the nucleus are achieved. The results of such research suggest that the processes are tightly controlled and are themselves critical components of gene regulation. Our long-term goal is understand how such processes occur in vivo and how their regulation dictates cell identity and cell-fate decisions in mammals.
To do so, our research program combines the robustness of mouse-genome editing and genetics with cutting-edge sequencing-based genomic techniques such as ATAC-seq, ChIP-seq, and Hi-C, as well as live-imaging approaches. We believe that the early mouse embryo is an ideal model system in which to determine how nuclear architecture is regulated in the context of an organism and how it impacts cell behavior and identity.
Fertilization is the ultimate reprogramming experiment, where two highly differentiated cells (oocyte and sperm) fuse to form a zygote with totipotent potential. This involves a massive rearrangement of epigenetic modifications, both at the level of the DNA and of the histones, and the activity of many transcriptional regulators. Our studies aim to understand how 3D chromatin structures are established during this period and how this impacts future developmental decisions.
Following fertilization and within a few cell divisions, the first cell lineages are established and different gene-expression programs are put into action. In mammals, the result is the formation of the blastocyst, a structure that contains three different cell types, each with a defined differentiation potential. The trophectoderm is responsible for forming the placenta, the primitive endoderm leads to the yolk sac, and the epiblast gives rise to all remaining embryonic tissues. We will build on decades of lineage-fate experiments and precisely characterized signaling pathways known to regulate early mouse development to understand the contribution of nuclear organization to gene regulation during these early cell fate decisions.
We are also interested in understanding not only how DNA organization impacts cell behavior, and ultimately animal development and health, but also the mechanisms through which DNA folding itself is established and regulated, and which proteins are involved in these processes. To broadly address these questions, we will employ several high-throughput technologies that we have established in the lab, in combination with genome-wide CRISPR screens. Ultimately, candidates identified this way will be fully characterized in vivo to stringently determine their impact on gene regulation during mammalian development.
Publications
- Oksuz O, Narendra V, Lee CH, Descostes N, LeRoy G, Raviram R, Blumenberg L, Karch K, Rocha PP, Garcia BA, Skok JA, Reinberg D. Capturing the onset of PRC2-mediated repressive domain formation. Mol Cell 2018;70:1149-1162.
- Raviram R, Rocha PP, Luo VM, Swanzey E, Miraldi ER, Chuong EB, Feschotte C, Bonneau R, Skok JA. Analysis of 3D genomic interactions identifies candidate host genes that transposable elements potentially regulate. Genome Biol 2018;19:216.
- Belver L, Yang AY, Albero R, Herranz D, Brundu FG, Quinn SA, Perez-Duran P, Alvarez S, Gianni F, Rashkovan M, Gurung D, Rocha PP, Raviram R, Reglero C, Cortes JR, Cooke AJ, Wendorff AA, Cordo V, Meijerink JP, Rabadan R, Ferrando AA. Gata3-controlled nucleosome eviction drives Myc enhancer activity in T-cell development and leukemia. Cancer Discov 2019;9(12):1774-1791.
Collaborators
- Sevinc Ercan, PhD, New York University, New York, NY
- Stefan Feske, MD, New York University School of Medicine, New York, NY
- Daniel Herranz, PharmD, PhD, Cancer Institute of New Jersey, Rutgers University, New Brunswick, NJ
- Timothy Petros, PhD, Unit on Cellular and Molecular Neurodevelopment, NICHD
- Danny Reinberg, PhD, New York University School of Medicine, New York, NY
- Achim Werner, PhD, Stem Cell Biochemistry Unit, NIDCR, Bethesda, MD
Contact
For more information, email pedro.rocha@nih.gov or visit https://irp.nih.gov/pi/pedro-rocha.


