Regulatory Small RNAs and Small Proteins

- Gisela Storz, PhD, Head, Section on Environmental Gene Regulation
- Aixia Zhang, PhD, Staff Scientist
- Philip P. Adams, PhD, Postdoctoral Fellow
- Aisha Burton Okala, PhD, Postdoctoral Fellow
- Andrew B. Kouse, PhD, Postdoctoral Fellow
- Sahar S. Melamed, PhD, Postdoctoral Fellow
- Medha V. Raina, PhD, Postdoctoral Fellow
- Lauren R. Walling, PhD, Postdoctoral Fellow
- Jeremy S. Weaver, PhD, Postdoctoral Fellow
- Mona Wu Orr, PhD, Postdoctoral Fellow
- Aoshu Zhong, PhD, Postdoctoral Fellow
- Jordan J. Aoyama, MD, Graduate Student
- Franchesca Uribe-Rheinbolt, BS, Postbaccalaureate Fellow
- Maxime Zamba-Campero, BS, Postbaccalaureate Fellow
The group currently has two main interests: (1) identification and characterization of small noncoding RNAs and (2) identification and characterization of small proteins of less than 50 amino acids. Both small RNAs and small proteins have been overlooked because they are not detected in biochemical assays, and the corresponding genes are missed by genome annotation and are poor targets for genetic approaches. However, both classes of small molecules are now being found to have important regulatory roles in organisms ranging from bacteria to humans.
Identification and characterization of small regulatory RNAs
During the past 20 years, we have carried out several different systematic screens for small regulatory RNA genes in Escherichia coli. The screens have included computational searches for conservation of intergenic regions and direct detection after size selection or co-immunoprecipitation with the RNA binding protein Hfq. We recently examined small RNA expression using deep sequencing to further extend our identification of small RNAs in a range of bacteria species.
A major focus for the group has been to elucidate the functions of the small RNAs that we and others identified. Early on, we showed that the OxyS RNA, whose expression is induced in response to oxidative stress, acts to repress translation through limited base-pairing with target mRNAs. We discovered that OxyS action is dependent on the Sm-like Hfq protein, which acts as a chaperone to facilitate OxyS RNA base-pairing with its target mRNAs. We now have also begun to explore the role of ProQ, a second RNA chaperone in E. coli.
It is clear that Hfq–binding small RNAs, which act through limited base-pairing, are integral to many different stress responses in E. coli and other bacteria as well as during the interaction between bacteria and bacteriophage [Reference 1]. For example, we showed that the Spot 42 RNA, whose levels are highest when glucose is present, plays a broad role in catabolite repression by directly repressing genes involved in central and secondary metabolism, redox balancing, and the consumption of diverse nonpreferred carbon sources. Similarly, we discovered that a Sigma(E)-dependent small RNA, MicL, transcribed from a promoter located within the coding sequence of the cutC gene, represses synthesis of the lipoprotein Lpp, the most abundant protein in the cell, to oppose membrane stress. We found that the copper sensitivity phenotype previously ascribed to inactivation of the cutC gene is actually derived from the loss of MicL and from elevated Lpp levels. This observation raises the possibility that other phenotypes currently attributed to protein defects can be attributed to deficiencies in unappreciated regulatory RNAs. Studies to determine the factors that direct the cleavage of MicL, and likely other small RNAs from the 3′ untranslated regions (UTRs) of mRNAs, showed that 3′ stem-loops are critical for the highly specific processing [Reference 2]. Most recently, while characterizing the response to limited magnesium, we found that the adjacently encoded MgrR small RNA and MgtS small protein both down regulate the pitA–encoded cation-phosphate symporter to increase intracellular magnesium levels [Reference 3].
In addition to small RNAs that act via limited base-pairing, we are interested in regulatory RNAs that act by other mechanisms. For example, early work showed that the 6S RNA binds to and modulates RNA polymerase by mimicking the structure of an open promoter. In a more recent study, we discovered that a broadly conserved RNA structure motif, the yybP–ykoY motif, found in the 5′-UTR of the mntP gene encoding a manganese exporter, directly binds manganese, resulting in a conformation that liberates the ribosome-binding site. Remarkably, we were able to recapitulate the effect of manganese-dependent activation of translation in vitro. We also found that the yybP–ykoY motif responds directly to manganese ions in Bacillus subtilis. The identification of the yybP–ykoY motif as a manganese-ion sensor suggests that the genes preceded by this motif and encoding a diverse set of poorly characterized membrane proteins have roles in metal homeostasis.
Further studies to characterize other Hfq–binding RNAs and their evolution, as well as regulatory RNAs that bind to other proteins such as ProQ [Reference 4] and act in ways other than base-pairing, are ongoing.
Identification and characterization of small proteins
In our genome-wide screens for small RNAs, we found that several short RNAs do indeed encode small proteins. The correct annotation of the smallest proteins is one of the biggest challenges of genome annotation, and there is limited evidence that annotated short ORFs encode synthesized proteins. Although these proteins have been largely missed, the few small proteins that have been studied in detail in bacterial and mammalian cells have been shown to have important functions in regulation, signaling, and cellular defenses [Reference 5]. We thus established a project to identify and characterize proteins of less than 50 amino acids.
We first used sequence conservation and ribosome-binding-site models to predict genes encoding small proteins of 16–50 amino acids in the intergenic regions of the model E. coli genome. We tested expression of these predicted as well as previously annotated small proteins by integrating the sequential peptide affinity tag directly upstream of the stop codon on the chromosome and assaying for synthesis using immunoblot assays. The approach confirmed that 20 previously annotated and 18 newly discovered proteins of 16–50 amino acids are synthesized. We recently carried out a complementary approach based on genome-wide ribosome profiling of ribosomes arrested in start codons to identify many additional candidates; the synthesis of 38 of these small proteins was confirmed by chromosomal tagging [Reference 6].
Many of the initially discovered proteins were predicted to consist of a single transmembrane alpha-helix and were found, by biochemical fractionation, to be in the inner membrane. Interestingly, assays of topology-reporter fusions and strains with defects in membrane-insertion proteins revealed that, despite their diminutive size, small membrane proteins display considerable diversity in topology and insertion pathways. Additionally, systematic assays for the accumulation of tagged versions of the proteins showed that many small proteins accumulate under specific growth conditions or after exposure to stress. We also generated and screened bar-coded null mutants and identified small proteins required for resistance to cell-envelope stress and acid shock.
We now are using the tagged derivatives and information about synthesis and subcellular localization and employing many of the approaches the group has used to characterize the functions of small regulatory RNAs to elucidate the functions of the small proteins. The combined approaches are beginning to yield insights into how the small proteins are acting in E. coli. We found that synthesis of a 42–amino acid protein, now denoted MntS, is repressed by high levels of manganese through MntR. The lack of MntS leads to reduced activities of manganese-dependent enzymes under manganese-poor conditions, while overproduction of MntS leads to very high intracellular manganese levels and bacteriostasis under manganese-rich conditions. These and other phenotypes led us to propose that MntS modulates intracellular manganese levels, possibly by inhibiting the manganese exporter MntP.
We also showed that the 31–amino acid inner-membrane protein MgtS (formerly denoted YneM), whose synthesis is induced by very low magnesium in a PhoPQ–dependent manner, acts to increase intracellular magnesium levels and maintain cell integrity upon magnesium depletion. Upon development of a functional tagged derivative of MgtS, we found that MgtS interacts with MgtA to increase the levels of this P-type ATPase magnesium transporter under magnesium-limiting conditions. Correspondingly, the effects of MgtS upon magnesium limitation are lost in an mgtA mutant, and MgtA overexpression can suppress the mgtS phenotype. MgtS stabilization of MgtA provides an additional layer of regulation of this tightly controlled magnesium transporter. Most recently, we found that MgtS also interacts with and modulates the activity of a second protein, the PitA cation-phosphate symporter, to further increase intracellular magnesium levels [Reference 3].
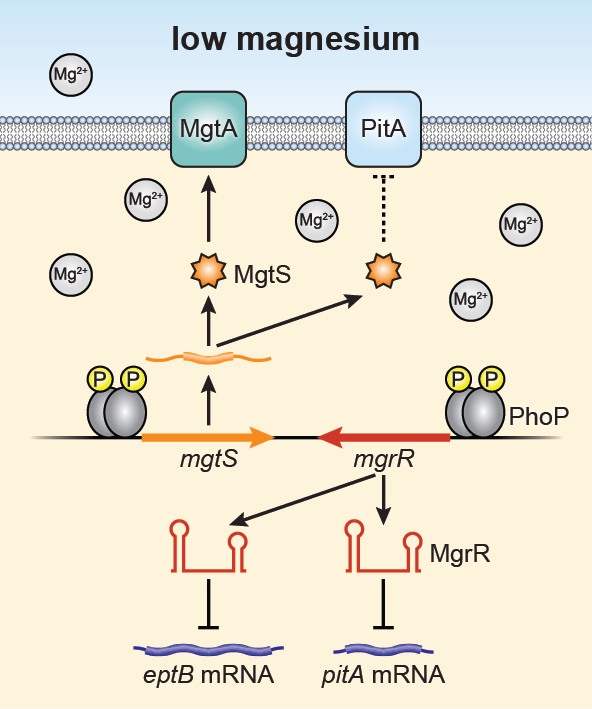
Click image to view.
Figure 1. Model for impact of convergently transcribed small protein and sRNA genes on intracellular Mg2+
In response to limiting Mg2+, the PhoQP two-component system induces the transcription of the mRNA encoding the 31-amino acid MgtS protein and MgrR sRNA. These small gene products were first shown to regulate the MgtA Mg2+importer and the eptB mRNA, respectively. Our recent studies show that they both also modulate the PitA phosphate symporter to increase intracellular Mg2+, pointing to a detrimental role of PitA under limiting Mg2+conditions.
We discovered the 49–amino acid inner-membrane protein AcrZ (formerly named YbhT), whose synthesis is increased in response to noxious compounds such as antibiotics and oxidizing agents, associates with the AcrAB–TolC multidrug efflux pump, which confers resistance to a wide variety of antibiotics and other compounds. Copurification of AcrZ with AcrB, in the absence of both AcrA and TolC, two-hybrid assays, and suppressor mutations indicate this interaction occurs through the inner-membrane protein AcrB. Mutants lacking AcrZ are sensitive to many, but not all, antibiotics transported by AcrAB–TolC. The differential antibiotic sensitivity suggests that AcrZ enhances the ability of the AcrAB–TolC pump to export certain classes of substrates. Detailed structural and mutational studies are now giving insight into how AcrZ changes AcrB activity.
This work, together with our ongoing studies of other small proteins and related findings by others in eukaryotic cells, supports our hypothesis that many small proteins act as regulators of larger membrane proteins.
Additional Funding
- NICHD Director's Investigator Award
Publications
- Altuvia S, Storz G, Papenfort K. Cross-regulation between bacteria and phages at a posttranscriptional level. Microbiol Spectr 2018;6:RWR-0027-2018.
- Updegrove TB, Kouse AB, Bandyra KJ, Storz G. Stem-loops direct precise processing of 3' UTR-derived small RNA MicL. Nucleic Acids Res 2019;47:1482-1492.
- Yin X, Wu Orr M, Wang H, Hobbs EC, Shabalina SA, Storz G. The small protein MgtS and small RNA MgrR modulate the PitA phosphate symporter to boost intracellular magnesium levels. Mol Microbiol 2019;111:131-144.
- Melamed S, Adams PP, Zhang A, Zhang H, Storz G. RNA-RNA interactomes of ProQ and Hfq reveal overlapping and competing roles. Mol Cell 2020;77(2):411-425.e7.
- Wu Orr M, Mao Y, Storz G, Qian S-B. Alternative ORFs and small ORFs: shedding light on the dark proteome. Nucleic Acids Res 2020;48(3):1029-1042.
- Weaver J, Mohammad F, Buskirk AR, Storz G. Identifying small proteins by ribosome profiling with stalled initiation complexes. mBio 2019;10:e02819-18.
Collaborators
- Shoshy Altuvia, PhD, The Hebrew University, Hadassah Medical School, Jerusalem, Israel
- Katarzyna J. Bandyra, PhD, University of Cambridge, Cambridge, United Kingdom
- Allen R. Buskirk, PhD, Johns Hopkins Medical Institute, Baltimore, MD
- Kai Papenfort, PhD, Ludwig-Maximilians-Universität, München, Germany
- Shu-Bing Qian, PhD, Cornell University, Ithaca, NY
- Svetlana A. Shabalina, PhD, National Center for Biotechnology Information, National Library of Medicine, Bethesda, MD
- Henry Zhang, PhD, Bioinformatics and Scientific Programming Core, NICHD, Bethesda, MD
Contact
For more information, email storz@helix.nih.gov or visit http://storz.nichd.nih.gov.


